
��1��37Cl�����ӽṹʾ��ͼ ��������ȵ���������SO2��SO3��������ԭ�Ӹ���֮
Ϊ ��
��2��20.6g NaR ����Na+0.2mol����NaR��Ħ������Ϊ ����R 8.0g ��NaR�����ʵ���Ϊ ��
��3���ڱ�״���£�4.8g����(CH4)��ռ�����Ϊ_________L�������״����________L����(H2S)������ͬ��Ŀ����ԭ�ӣ�
��4����100gŨ��Ϊ18 mol��L��1���ܶ�Ϊ��(g��cm�C3)��Ũ�����м���һ������ˮϡ�ͳ�9mol��L��1�����ᣬ�����ˮ����� 100mL�������������������������
��1��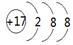 5: 6
5: 6
��2��103 g/mol����λ��д���÷֣��� 0.1 mol
��3��6.72�� 13.44
��4����
���������������1��Cl?Ϊ18���ӣ������������Ϊ8��������ȵ���������SO2��SO3��������ԭ�Ӹ���֮Ϊm��64��2��m��80��3=5:6��
��2��Na+Ϊ0.2mol����NaRΪ0.2mol��M(NaR)=20.6g��0.2mol=103g/mol����NaR��Ħ�������ɵ�M(R)=80g/mol����n(NaR)=n(R)=8.0g��80g/mol=0.1mol��
��3����������Ħ��������м��㣬V(CH4)=4.8g��16g/mol��22.4L/mol=6.72L��4.8g��16g/mol��4=V(H2S)��22.4L/mol��2,�ɵ�V(H2S)=13.44L��
��4����18 mol��L-1H2SO4ϡ��Ϊ9mol��L-1��Ӧʹ��Һ�����Ϊԭ����2������Ϊ������Һ���ܶ�����Ũ�ȵļ�С����С��ϡ�ͺ��������Һ����С��ԭ��Һ��2�������Լ���ˮ�����С��100ml��
���㣺���⿼�黯ѧ�����ѧ���㡣


 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1.66g̼���ƺ��������ƵĹ���������ȫ����ˮ�����ϡ��Һ��Ȼ�������Һ����μ���1mo1/L�����ᣬ�������������������CO2���������״������ϵ��ͼ��ʾ��
��ʾ��Na2CO3�������Ƿֲ����еģ���һ��ΪNa2CO3+HCl=NaHCO3+NaCl���ڶ���ΪNaHCO3+HCl=NaCl+H2O+CO2����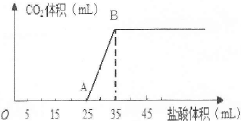
��ش��������⣺
��1��OA����������Na2CO3��NaOH�����ķ�Ӧ����Ӧ����Һ�е������� (�ѧʽ)��
��2��������B��ʱ������CO2�����Ϊ mL(��״��)��
��3������ԭ�������Na2CO3������������(д��������̣��������С�����һλ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�ᾧˮ���ﺬ�����������Ӻ�һ�������ӡ���ȡ����������Ϊ45.3 g�ĸýᾧˮ����ֱ��Ƴ���Һ��������һ����μ���NaOH��Һ�����ֿ�ʼ��Һ�г��ְ�ɫ�����������ࣻһ��ʱ����������ݳ����������д̼�����ζ����ʹʪ��ĺ�ɫʯ����ֽ���������Ⱥƿ��ռ���2.24 L�����壨��״����������ɫ�������ٲ�������ʧ����һ����μ���Ba(OH)2��Һ����ʼ�������ƣ����������а�ɫ���������ˣ���ϡ���ᴦ���������ϴ�Ӻ���õ���ɫ����46.6 g��
��ش��������⣺
��1���ýᾧˮ�����к��е������������ǣ��� ������ ������
��2����ͨ������ȷ���ýᾧˮ����Ļ�ѧʽΪ���� ������
��3����������������Һ�м����Ba(OH)2��Һ�����ʵ���Ũ��Ϊ2.0 mol��L-1��
�ټ���Ba(OH)2��Һ�������ó����������ʵ��������Ӧ�����ӷ���ʽΪ���� ������
��������75 mL��Ba(OH)2��Һ����õ��ij�������Ϊ������ �� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ��������Ҫ����500 mL 0.10 mol·L-1��NaOH��Һ����ʵ��ش��������⡣
��1����������ƽ����NaOH��̬ g������NaOH������ע�������������⣺����ΪNaOH���и�ʴ�ԣ����Գ���ʱ��Ӧѡ�� ʢװNaOH���壻�ڳ�������Ѹ�٣�Ŀ���Ƿ�ֹ ��
��2��ʵ������Ҫ��������������ƽ��ҩ���⣬����Ҫ�IJ��������У� �� �� �� ��
��3�����в����������Ƶ���ҺŨ��û��Ӱ����� ��
| A������ʱ���ӿ̶��� |
| B�����ձ����ܽ�����Һ����ע������ƿ��Ȼ������������ˮ���̶��� |
| C��ҡ�ȶ��ݺ��ý�ͷ�ι�������ƿ�еμ�����ˮ���̶��� |
| D��������Һǰ������ˮ��ϴ����ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����m gij���壬������ԭ�ӷ��ӹ��ɣ�����Ħ������ΪM g��mol��1����
��1������������ʵ���Ϊ____ ____��
��2����������������ԭ������Ϊ________����
��3���������ڱ�״���µ����Ϊ________L��
��4������������1 Lˮ��(�����Ƿ�Ӧ)������Һ�����ʵ���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
| ���� ����ʽ��HCl ��Է���������36.5,�ܶȣ�1.19 g��cm��3 HCl������������36.5% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ�����������գ�
��1����ϩ�����ۺϷ�Ӧ�ķ���ʽΪ��
��2������������Ӧ�Ļ�ѧ����ʽΪ��
��3���Ҵ���ͭ���������´������ķ���ʽΪ��
��4��д��CH3C18O16OH��C2H518OH����������Ӧ�ķ���ʽ����
��5��д�����������������۷�����Ӧ�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ʵ�����CO��CO2������Oԭ�Ӹ���֮�� ��Cԭ����֮�� �����ߵ�����֮�� ����ͬ��ͬѹ�µ����֮�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������2.05 gij�ۺ�����ĸ��ε���ҺA�뺬1.20 g̼���ε���ҺB��ϣ�ǡ����ȫ��Ӧ������1.25 g��ɫ����C������ȥ����C����Һ�������õ���ɫ����D����������Dʱ��D�ֽ�ֻ��������̬���ʵĻ�����0 �桢1��105 Pa�£������Ϊ0.56 L�����õ�0.90 gҺ̬ˮ����һ����̬����Ϊ��̬������R2O���Իش�
(1)��ɫ����C�����ʵ���Ϊ________mol��
(2)A��Ħ������Ϊ__________��B��Ħ������Ϊ__________��
(3)R2O��H2O�����ʵ���֮��Ϊ__________������D������Ϊ________��D��Ħ������Ϊ________��R2O����Է�������Ϊ________��R2O�Ļ�ѧʽ��____________��
(4)д��A��B��ϵĻ�ѧ����ʽ_____________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com