
ʵ��������Ҫ����500 mL 0.10 mol·L-1��NaOH��Һ����ʵ��ش��������⡣
��1����������ƽ����NaOH��̬ g������NaOH������ע�������������⣺����ΪNaOH���и�ʴ�ԣ����Գ���ʱ��Ӧѡ�� ʢװNaOH���壻�ڳ�������Ѹ�٣�Ŀ���Ƿ�ֹ ��
��2��ʵ������Ҫ��������������ƽ��ҩ���⣬����Ҫ�IJ��������У� �� �� �� ��
��3�����в����������Ƶ���ҺŨ��û��Ӱ����� ��
| A������ʱ���ӿ̶��� |
| B�����ձ����ܽ�����Һ����ע������ƿ��Ȼ������������ˮ���̶��� |
| C��ҡ�ȶ��ݺ��ý�ͷ�ι�������ƿ�еμ�����ˮ���̶��� |
| D��������Һǰ������ˮ��ϴ����ƿ |
��1��2.0g С�ձ� ���⡣��������ն�����̼��ˮ��
��2��500ml����ƿ���ձ�����ͷ�ιܡ�����������3��D�� ����1�֣�
���������������1���и�ʴ�Ե����ʳ�����Ӧ���ڲ��������У��ձ���������Ⱦ��ɣ���3��A�����ӿ̶��ᵼ������ƿ����Һ���ƫС����ɽ��Ũ��ƫ�ߣ�B�����������ܽ���ȣ�����ȴ�����º���ע������ƿ�У������Ũ��ƫ�ߣ�C��ҡ�ȶ��ݺ�����������ƿ�еμ�����ˮ������Ũ��ƫС��
���㣺������Һ�������й����⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һֻ���汻������10.2 g������������A������1 L 1.5 mol��L��������������Һ�У���һ��ʱ���ȡ����������Һ��������12.6�ˣ�����Һ��Ϊ����Һ�������Ϊ1 L���ٰ���һֻ����Ҳ������һ������������������B������1 L 0.9 mol��L��������Һ�У�����һ��ʱ��ȡ��������������25.8�ˣ���Һ��������25.2�ˣ�����Һ��Ϊ����Һ�������Ϊ1 L���ش��������⣺
��1������Һ������ ��д��ѧʽ���������ʵ����� ;
��2������Һ������ ��д��ѧʽ���������ʵ����� ;
��3������������Һ��Ӧ����ʹ�μӵ�����С������������࣬Ӧ�� ��Һ ����ס��ң�L �μӵ� ��Һ�У���ס��ң���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ʵ����Ǹ��л�ѧ�г��õ�������������������й������ݵļ��㡣
��1��0.2 g H2���и�______Hԭ�ӡ�
��2����״���£�������ͬ��ԭ������CO��CO2�����֮��Ϊ______��
��3��100 mL ijAl2��SO4��3��Һ�У�n��Al3+��="0.20" mol��������c��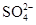 ��= ______mol/L��
��= ______mol/L��
��4����9.5 gij���۽������Ȼ����к�0.2 mol Cl-�����Ȼ����Ħ������Ϊ______���ý���Ԫ�ص����ԭ������Ϊ______��
��5����״����6.72 L CO��һ������ Fe2O3ǡ����ȫ��Ӧ������Fe��CO2����ʣ����������Ϊ______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ����Ҫ����1mol/L��ϡ����250mL���ش��������⣺
��1����Ҫ98%�ܶ�Ϊ1.84g/cm3��Ũ���� mL
��2������ʱ������ʹ�õ������� ���������� (�����) ��ȱ�ٵ������� ������ ��
���ձ�����100 mL��Ͳ������20 mL��Ͳ ��1000 mL����ƿ ��250 mL����ƿ����������ƽ(������) �߲�����
��3������ʱ����ʵ�������õ��������������÷ֱ��� �� ��
��4�����ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ���ƫ�͡�����Ӱ�족��
��û��ϴ���ձ��Ͳ���������������������������
�������ˮ�����˿̶��ߣ�ȡ��ˮʹҺ��ǡ�õ��̶��ߡ�����������������
������ƿû�и������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�����һ�������ۺ����۵Ļ������һ�����ijŨ�ȵ�ϡ�����ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���5 mol��L��1��NaOH��Һ������NaOH��Һ�����(mL)������ij��������ʵ�����ϵ��ͼ��ʾ����
��1��B��A�IJ�ֵΪ________mol��
��2��ԭ������Һ�к���������ʵ���Ϊ________mol��
��3�����ۺ����۵Ļ���������������۵����ʵ���֮��Ϊ________��
��4��д�������Ũ�����ᷴӦ�����ӷ���ʽ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��37Cl�����ӽṹʾ��ͼ ��������ȵ���������SO2��SO3��������ԭ�Ӹ���֮
Ϊ ��
��2��20.6g NaR ����Na+0.2mol����NaR��Ħ������Ϊ ����R 8.0g ��NaR�����ʵ���Ϊ ��
��3���ڱ�״���£�4.8g����(CH4)��ռ�����Ϊ_________L�������״����________L����(H2S)������ͬ��Ŀ����ԭ�ӣ�
��4����100gŨ��Ϊ18 mol��L��1���ܶ�Ϊ��(g��cm�C3)��Ũ�����м���һ������ˮϡ�ͳ�9mol��L��1�����ᣬ�����ˮ����� 100mL�������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Ϊ98%���ܶ�Ϊ1��84g��cm��3��Ũ��������l mol��Lϡ����100mL������������¸�����
������Ͳ��ȡ mLŨ����
��ϴ���ձ��Ͳ�����2-3�Σ���ϴ��Һת������ƿ��
�۽�ϡ�͡���ȴ�������ת��100 mL����ƿ��
�ܽ�Ũ���ᵹ��ʢ��ˮ���ձ���ϡ�͡���ȴ
�ݼ�ˮ��Һ��ӽ��̶���1��2cm�������ݣ�ҡ��
���������գ�
��1���ڢٲ�������Ӧ����Ͳ��ȡ mLŨ���Ӧѡ�� mL��Ͳ����5��10��50����
��2����ʵ���õ��Ļ������������ձ�����Ͳ������������ȱ�ٵ������� ��
��3����ȷ�IJ���˳���ǣ��������д�� ��
��4�����в�����ʹʵ��Ũ��ƫ�ߣ�ƫ�ͻ��Dz��䣬����д��
1����ҡ�Ⱥ�Һ����ڿ̶��ߣ�û���ټ�ˮ��
2������Һ�����У���С�Ľ���������Һ��
3��������ʱ��������ӿ̶��ߣ����Ƶ�����Ũ�Ƚ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��2.3����Ͷ�뵽ˮ�����з�̪���У���Ӧ��������Һ��0.1������
��1����Ӧ�����У����Է����Ƹ���ˮ�棬˵���Ƶ��ܶ� ������ڡ���С�ڡ�����
�ڡ���ˮ���ܶȣ���Һ����� ɫ��
��2����ѧ��Ӧ����ʽ�� ��
��3���������Һ�����ʵ���Ũ���Ƕ��٣���д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Իش��������⣺
��1����֪24��A��40��Bǡ����ȫ��Ӧ����0.4molC��32��D����C��Ħ������Ϊ ��
��2����ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
�ٸ�Ũ������HCl�����ʵ���Ũ��Ϊ mol/L��
����ʵ��������450mL1.19 mol/L��ϡ���ᣬ���ø�Ũ����________ mL,����ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������_______________(����������)��
��ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��____1.19mol/L (����ڡ������ڡ���С�ڡ�����ͬ)����������Һ��ת��������ƿʱ����������������������ҺŨ��_______1.19mol/L��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com