



 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������֣����ᣩ��������������̼������Һ����������ռ������ |
| B���Ҵ���ˮ��������������ʯ�ң������ռ������ |
| C���������ӣ�������ˮ�������˳�ȥ���� |
| D���������������ᣩ���ӱ���̼������Һ���������Һ����ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
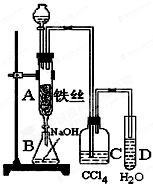
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����AgNO3��Һ����NaCl��Na2SO4 |
| B����ȥ������������,�������м���������NaOH��Һ����� |
| C������ˮ��CCl4�����Һ©��,����,�ɽ�����ȡ��CCl4�� |
| D�����Ҵ���Ũ�����Ʊ���ϩʱ������ˮԡ���ȿ��Ʒ�Ӧ���¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ˮ | B��FeCl3��Һ |
| C������Cu(OH)2����Һ | D��NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����mL) | 24.00 | 24.10 | 22.40 | 23.90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ױ������� | B�����ۣ������ǣ� | C�������ᣨ���ӣ� | D���屽���壩 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
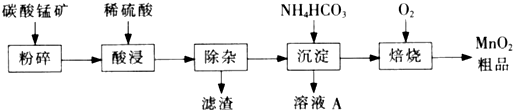 �й��������↑ʼ�����ͳ�����ȫ��pH���±���
�й��������↑ʼ�����ͳ�����ȫ��pH���±���| �������� | Al��OH��2 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Pb��OH��2 | Mn��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com