
����Ŀ��Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�顣
[����ʽ��ȷ��]
(1)���л���A�����������г��ȼ�գ�ʵ��������5.4 g H2O��8.8 g CO2����������6.72 L(��״����)����������и�Ԫ�ص�ԭ�Ӹ�������__________��
(2)�����Dzⶨ�л����������Է�������Ϊ46��������ʵķ���ʽ��__________��
(3)���ݼۼ����ۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ_____________________��
[�ṹʽ��ȷ��]
(4)���ⶨ���л���A�ĺ˴Ź���������ͼ��ʾ����A�Ľṹ��ʽΪ_____________��
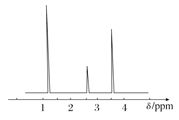
[����ʵ��]
(5)A��Cu�����¿ɱ���������B���仯ѧ����ʽΪ___________________________��
(6)A��ͨ����ʳ��һ���������Ƶã�����ʳ�Ƶõ�A��һ���¶����ܱմ��棬��Ϊ����һϵ�еĻ�ѧ�仯����ø����㡣��д�����һ����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________��
���𰸡� N(C)��N(H)��N(O)��2��6��1 C2H6O CH3CH2OH��CH3OCH3 CH3CH2OH 2CH3CH2OH��O2![]() 2CH3CHO��2H2O CH3COOH��CH3CH2OH
2CH3CHO��2H2O CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
��������(1)�������У�n(H2O)��0.3 mol������n(H)��0.6 mol��n(CO2)��0.2 mol������n(C)��0.2 mol������ԭ���غ���n(O)��n(H2O)��2n(CO2)��2n(O2)��0.3 mol��2��0.2 mol��2��![]() ��0.1 mol��������ʸ�Ԫ��ԭ�Ӹ�����N(C)��N(H)��N(O)��n(C)��n(H)��n(O)��2:6:1��(2)��(1)��֪���л����ʵ��ʽΪC2H6O��������л���ķ���ʽΪ(C2H6O)m��������ͼ֪����Է�������Ϊ46����46m��46����m��1���������ʽΪC2H6O��(3)��A�ķ���ʽΪC2H6O��֪AΪ���ͻ�������Ʋ���ṹΪCH3CH2OH��CH3OCH3��(4)����A�ĺ˴Ź�������ͼ��֪��A�����ֲ�ͬ���͵�Hԭ�ӣ�CH3OCH3ֻ��һ�����͵�Hԭ�ӣ���A�Ľṹ��ʽΪCH3CH2OH��(5)�Ҵ���Cu�����¿ɱ�����������ȩ���仯ѧ����ʽΪ��2CH3CH2OH��O2
��0.1 mol��������ʸ�Ԫ��ԭ�Ӹ�����N(C)��N(H)��N(O)��n(C)��n(H)��n(O)��2:6:1��(2)��(1)��֪���л����ʵ��ʽΪC2H6O��������л���ķ���ʽΪ(C2H6O)m��������ͼ֪����Է�������Ϊ46����46m��46����m��1���������ʽΪC2H6O��(3)��A�ķ���ʽΪC2H6O��֪AΪ���ͻ�������Ʋ���ṹΪCH3CH2OH��CH3OCH3��(4)����A�ĺ˴Ź�������ͼ��֪��A�����ֲ�ͬ���͵�Hԭ�ӣ�CH3OCH3ֻ��һ�����͵�Hԭ�ӣ���A�Ľṹ��ʽΪCH3CH2OH��(5)�Ҵ���Cu�����¿ɱ�����������ȩ���仯ѧ����ʽΪ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O��(6)����ʳ�Ƶõ��Ҵ��ܱձ���ʱ���ֻ���������CH3COOH���Ҵ������ᷢ��������Ӧ�����˾�����ζ��������������������Ӧ�ķ���ʽΪ��CH3COOH��CH3CH2OH
2CH3CHO��2H2O��(6)����ʳ�Ƶõ��Ҵ��ܱձ���ʱ���ֻ���������CH3COOH���Ҵ������ᷢ��������Ӧ�����˾�����ζ��������������������Ӧ�ķ���ʽΪ��CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��


 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��700��ʱ�����ݻ�Ϊ2L���ܱ������г���һ������CO��H2O,������Ӧ��CO��g��+ H2O��g��![]() CO2 (g)+ H2��g����Ӧ�����вⶨ�IJ������ݼ��±�������t2>t1��:
CO2 (g)+ H2��g����Ӧ�����вⶨ�IJ������ݼ��±�������t2>t1��:
��Ӧʱ��/min | n��CO��/mol | n(H2O)/mol |
0 | 1.20 | 0.60 |
t1 | 0.80 | |
t2 | 0.20 |
����˵����ȷ���ǣ� ��
A. ��Ӧ��t1min�ڵ�ƽ������Ϊ![]()
B. ���������������䣬��ʼʱ�������г���0.60molCO��1.20 molH2O������ƽ��ʱn(CO2)�� 0.40mol
C. ���������������䣬��ƽ����ϵ����ͨ��0.20molH2O����ԭƽ����ȣ��ﵽ��ƽ��ʱH2Oת��������
D. �¶�����800�棬������Ӧƽ�ⳣ��Ϊ0.64��������ӦΪ���ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ��ú������
(1)��ˮ����ͨ�����ȵ�̿���ɲ���ˮú������ӦΪC(s)��H2O(g)CO(g)��H2(g)����H����131.3 kJ��mol��1��
�ٸ÷�Ӧ�ڳ�����_______(������������������)�Է����С�
��һ���¶��£���һ���ݻ��ɱ���ܱ������У�����������Ӧ���������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����____(����ĸ����ͬ)��
a�������е�ѹǿ����
b��1 mol H��H�����ѵ�ͬʱ������2 mol H��O��
c��c(CO)��c(H2)
d���ܱ��������ݻ����ٸı�
(2)����ͬ����CO(g)��H2O(g)�ֱ�ͨ�����Ϊ2 L�ĺ����ܱ������У����з�ӦCO(g)��H2O(g)CO2(g)��H2(g)���õ������������ݣ�
ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
H2O | CO | H2 | CO | |||
1 | 650 | 2 | 4 | 1.6 | 2.4 | 6 |
2 | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
3 | 900 | a | b | c | d | t |
��ʵ��1�дӷ�Ӧ��ʼ��ƽ����CO2��ʾ��ƽ����Ӧ����Ϊv(CO2)��________(ȡС�������λ����ͬ)��
�ڸ÷�Ӧ������ӦΪ________(
(3)Ŀǰ��ҵ����һ�ַ�������CO2�������״���һ�������·�����Ӧ��CO2(g)��3H2(g)CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯�������Ϊ1 L�ĺ����ܱ������У�����1 mol CO2��3 mol H2���ﵽƽ������д�ʩ����ʹc(CH3OH)�������________��

a�������¶�
b������He(g)��ʹ��ϵѹǿ����
c����H2O(g)����ϵ�з������
d���ٳ���1 mol CO2��3 mol H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ɫ��ҺX����Na+��Mg2+��Ba2+��Al3+��AlO2-��MnO4-��CO32-��SO42-�е�������������ɣ�ȡ��Һ������������ʵ����

��1������A�ijɷ���_______���ѧʽ����ͬ�� ������B�ijɷ���_______��
��2��X��Һ��һ�����ڵ�������______________��
��3��д��������з�����Ӧ���������ӷ���ʽ��______________��
��4��д����������γɰ�ɫ���������ӷ���ʽ��______________��
��5��ͨ������ʵ�飬��ҺX�в���ȷ���Ƿ���ڵ�������______________��ֻҪ���һ���ĺ���ʵ��Ϳ���ȷ���������Ƿ���ڣ��÷�����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ѧ�м��ֳ������ʵ�ת����ϵ����ͼ��ʾ

��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫ���壮��ش��������⣺
��1�����ɫ������F����ֱ����С�ķ�Χ��________.
��2��A��B��H�Ļ�ѧʽ��A__________��B__________�� H________.
��3����H2O2���ӵĵ���ʽΪ______________��
��д��C��������Һ��˫��ˮ��Ӧ�����ӷ���ʽ�� _______________________��
��4��д������E�������ӵ�ʵ�鷽��������_______________________��
��5����C��Һ�м�����C�����ʵ�����Na 2 O 2 ��ǡ��ʹCת��ΪF��д���÷�Ӧ�����ӷ���ʽ��____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ�������µ�������ͨ���������û����ֿ����������������ͬ�����ҳ���һ����NO�����ҳ���һ����O2����ǡ��ʹ�������������ܶ���ͬ��������ʹNO��O2��ַ�Ӧ�һָ������£���Ӧ����:2NO+O2=2NO2��������NO2ת��ΪN2O4�������ж���ȷ����
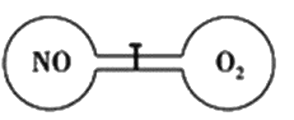
A. ��Ӧǰ�����ҵ�ѹǿ��ͬ
B. ��Ӧ�������ڵ��ܶ��뷴Ӧǰ����ͬ
C. ��ʼʱ���������еķ�������ͬ
D. ��Ӧ����������O2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ������������Ҫ�ɷ�Ϊ������������������������ȡ���Ĺ�����ͼ��ʾ��

��ش�
��1���Լ�1Ϊ____________���ѧʽ�����١���ת��������____________����������ࣻ
��2������B�Ļ�ѧʽΪ__________�������ʳ������ڽ���ұ�����⣬��������_____________��
��3�����F����ת��6mol����ʱ�����Ƶ��� _____________g��
��4�����������У�������E����ѭ��ʹ���⣬������ѭ��ʹ�õ�������__________���ѧʽ����
��5�����з�����Ӧ�����ӷ���ʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и����е����������Ӧʱ�����ı䷴Ӧ����(�¶ȡ���Ӧ��������)����ѧ��Ӧ�ı��ʲ����ı����( )
A. Na��O2 B. Cu������ C. Na2O2��CO2 D. ľ̿(C)��O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������װ����ȡ������������������ʵ�顣�ش��������⣺
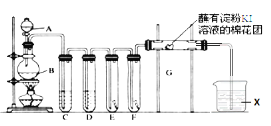
(1)A��ʢ��Ũ���ᣬB��ʢ��MnO2��д����Ӧ�����ӷ���ʽ__________________________��
(2)E��Ϊ��ɫ�ɲ�����F��Ϊ��ɫʪ�������ɹ۲쵽��������________________________���Ա�E��F������IJ���ɵó��Ľ��ۼ�������________________________________��
(3)�Թ�C��D���Լ���Ϊ_____________________________��
(4)װ��G��������Ӧ�����ӷ���ʽΪ_______________________________��
(5)�����ӷ���ʽ��ʾ�Լ�X������_________________________��
(6)��ͥ�г�������Һ(��Ҫ�ɷ�NaClO)������(��Ҫ�ɷ�����)���������ijƷ������Һ��װ��˵������ͼ��

����������ͬʱʹ����������ж���������д����Ӧ�����ӷ���ʽ__________________��
�������ܱձ�������ԭ��____________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com