
����Ŀ����ѧ����������
��1������β���dz��п�����Ⱦ��Դ֮һ������β���к���CO��NO���к����ʡ�ͨ������Ĵ�װ�ã�����ʹCO��NO���Ӧ���������ȶ�������N2��CO2����Ӧ�Ļ�ѧ����ʽ��_____________������Ӧ����1molN2��ת�Ƶĵ�����ĿΪ_______________��(NA��ʾ�����ӵ�������ֵ)
��2��Cl2+2NaOH=NaCl+NaClO+H2O�ǹ�ҵ����84����Һ��ԭ������������ѧ����ʽ��Ϊ���ӷ���ʽ������˫���ŷ���������ת�Ƶķ������Ŀ��_________________
��3����һ�����¶Ⱥ�ѹǿ�£�1���X2 (��)��3���Y2 (��)��������2����������û�����Ļ�ѧʽ��_____________��
���𰸡�2CO + 2NO![]() N2+2CO2 4NA �� XY3��Y3X
N2+2CO2 4NA �� XY3��Y3X
��������
��1��CO��NO���Ӧ���������ȶ�������N2��CO2����Ӧ�Ļ�ѧ����ʽ��2CO + 2NO![]() N2+2CO2��2NO
N2+2CO2��2NO![]() N2��Ԫ�صĻ��ϼ���+2��0�ۣ�����Ӧ����1molN2��ת�Ƶĵ�����ĿΪ2
N2��Ԫ�صĻ��ϼ���+2��0�ۣ�����Ӧ����1molN2��ת�Ƶĵ�����ĿΪ2![]() 2=4mol,��Ϊ4NA���𰸣�2CO + 2NO
2=4mol,��Ϊ4NA���𰸣�2CO + 2NO![]() N2+2CO2 4NA��
N2+2CO2 4NA��
��2��Cl2+2NaOH = NaCl+NaClO+H2O�����绯��Ӧ�������ӷ���ʽΪ![]() ������˫���ŷ���������ת�Ƶķ������Ŀ
������˫���ŷ���������ת�Ƶķ������Ŀ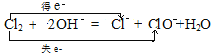 ���𰸣�
���𰸣�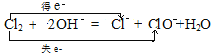 ��
��
(3)��û����ﻯѧʽΪXaYb���ɰ����ӵ�����֪X2+3Y2=2 XaYb�����������غ㶨�ɣ��ã�2=2a��a=1��2b=6��b=3����������ΪXY3���𰸣�XY3��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.ʵ��������ͼװ����ȡ���о�SO2�����ʡ�
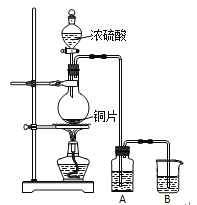
���������գ�
��1��Բ����ƿ�з�Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��2����A��ʢ��Ʒ����Һ��������֤��SO2������______________����Ҫ��֤SO2�Ļ�ԭ�ԣ�A��Ӧʢ��_________��Һ��B��Ӧʢ��_________��Һ��
II.Ϊ�ⶨ��Ӧ�����Һ�в���������Ũ�ȣ�ʵ��С���������̽����
��3�����飺ȡ1 mL��ӦҺ��ˮϡ����100 mL����ȡ10 mLϡ��Һ������п������ͼװ�ý��вⶨ���ڶ�ȡ��Ӧǰ����������Һ�����ʱӦ�ȵ���ˮ������������Һ��ȸߣ�����������Ŀ����________��
ʵ���÷�Ӧ���ɵ��������Ϊ22.4 mL���ѻ��㵽��״����������ԭ��ӦҺ�в���������Ũ��Ϊ______mol/L��
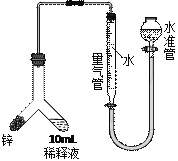
��4�����飺��ͨ������������BaCl2��Һ���ⶨ���ɵ�BaSO4�����������ﵽʵ��Ŀ�ġ������˼·�����У�������_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij��Br����ˮ����ȡBr2�Ĺ��̰��������ˡ���������ȡ����ѡ�������ȡ����������Ȳ��衣��֪��
���� | Br2 | CCl4 | ��ʮ���� |
�ܶ�/g��cm-3 | 3.119 | 1.595 | 0.753 |
�е�/�� | 58.76 | 76.8 | 215~217 |
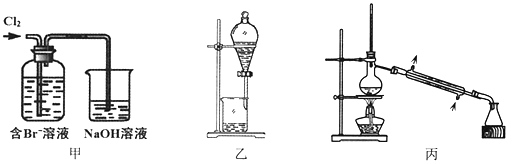
����˵������ȷ����
A. ��װ����Br�������ķ�ӦΪ��2Br-+ Cl2 = Br2 + 2Cl-
B. ��װ����NaOH��Һÿ����0.1mol Cl2��ת��0.1mol e��
C. ����װ�ý�����ȡ���ܽ�Br2���л������²�
D. �ñ�װ�ý����������ռ�������Br2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯�����ڹ�ũҵ����������Ҫ��;����Ҳ������������Ԫ�ء�
��1����(34Se)�����ڱ���λ�����·���������ԭ�ӽṹʾ��ͼ_____________��
��2������Ԫ�ص��ʾ�����H2��Ӧ����H2X����ԭ�ӽṹ����ԭ��_______________��
��3��298K��1.01��l0-5Pa��O2��S��Se��Te�ֱ���H2���ϵķ�Ӧ��������ͼ��ʾ��д��Se��H2���ϵ��Ȼ�ѧ��Ӧ����ʽ__________________��
��4�����Դӵ�⾫��ͭ������������ȡ����ͨ����ѧ���յõ��������ƵȺ������ʡ������£�Se(��)��Һ�и���ֵ����ʵ���������pH�仯������ͼ��
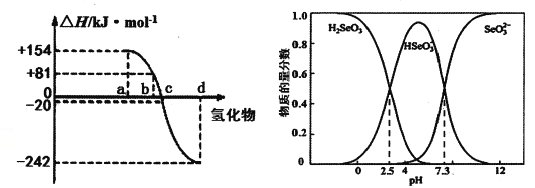
������������Һ����NaOH��Һ��pH =5���ù�������Ҫ��Ӧ�����ӷ���ʽ________��
����pH<0�����Ի����£���Se(��)��ϵ��ͨ��SO2�Ƶõ���Se�Ļ�ѧ����ʽ��_______��
������˵����ȷ����____������ĸ��ţ���
a��NaHSeO3��Һ������
b��pH=8ʱ����Һ�д���c(HAsO3-)+2c(SeO32-)+c(OH-)=c(H+)
c����Na2SeO3��Һ�У�c(SeO32-��c(HSeO3-)> c(H2SeO3)
�������£�H2SeO3�ĵڶ�������ƽ�ⳣ��ΪK2������K2=________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��PVAc��һ�־��������Ե���֬���ɺϳ���Ҫ�߷��Ӳ���M���ϳ�·�����£�
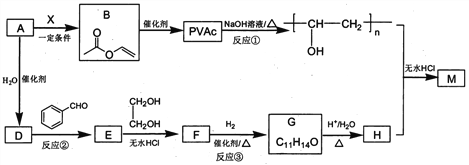
��֪��R��R�@��R�@�@ΪHԭ�ӻ�����
I. R'CHO+ R"CH2CHO![]()
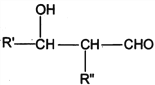
![]()
![]()
II. RCHO+![]()
![]()
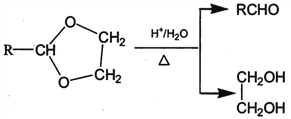
��1����״���£�4.48L��̬��A��������5.2g����A�Ľṹ��ʽΪ___________________��
��2����֪A��BΪ�ӳɷ�Ӧ����X�Ľṹ��ʽΪ_______��B�й����ŵ�������_________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ______________________��
��4��E��ʹ������Ȼ�̼��Һ��ɫ����Ӧ�ڵķ�Ӧ�Լ���������_______________________��
��5����Ӧ�۵Ļ�ѧ����ʽΪ____________________________��
��6����E��F��G��H��ת�������У��Ҷ�����������__________________________��
��7����֪M�������г������⣬��������Ԫ��״�ṹ����M�Ľṹ��ʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�鷽�������������������Ҵ����������
A. ��������ˮ B. ����̼��������Һ
C. �μ���ɫʯ����Һ D. �μ����Ը��������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨ���ȷ�Ӧʵ���������������ijɷ֣�ijѧ����ȤС�����ʵ��������ͼ��ʾ��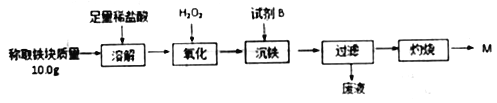
�����������↑ʼ��������ȫ������pH���±���ʾ��
Fe2+ | Fe3+ | Al3+ | Mg2+ | |
��ʼ����ʱ��pH | 7.5 | 2.8 | 4.2 | 9.6 |
������ȫʱ��pH | 9.0 | 4.0 | 5 | 11 |
(1)ȡ�ܽ�����õ�����Һ�������еμ�KSCN��Һ��������Һ��Ѫ��ɫ���������������ԭ���˿��ܻ���û��Ӧ����������⣬����һ��ԭ����_______________��
(2)��������Ӧ������ҺpH�ķ�Χ��Ϊ________���Լ�B ѡ��______��(�����)
a.ϡ���� b.������ c.MgCO3���� d.��ˮ
(3)��֪������Fe(OH)3��Ksp=1.1��10-36��������ȫ����ʱ��Һ��c(Fe3+)=_____mol/L��
(4)�������̵õ��Ĺ���M����_____(��ܡ����ܡ�)����������н�������������ԭ����________________��
(5)����С����õζ��ķ�ʽ�ⶨ�����顱�����ĺ���(��Ԫ��ֻ����Fe��Fe2O3)���ⶨ��ʽ���£�
I.ȡ10g�����顱���飬������������������Һ��ַ�Ӧ�����ˡ�ϴ�ӡ�����õ�8.88g���塣������������������ܽ⣬���200mL��ҺA��
II.ȡ��10mL ��ҺA ����ƿ���ȼ���������SnCl2��Һ����Fe3+ȫ��ת��ΪFe2+����ȥ������Sn2+��
III.����һ��������������ᣬ���μ�ָʾ����������ƿ�еμ�0.1000mol/LK2Cr2O7��Һ��ǡ����ȫ��Ӧʱ��������12.50mL K2Cr2O7��Һ��������Ӧ:Cr2O72-+6Fe2++14H+==2Cr3++6Fe3++7H2O
�ٲ���I�м�����������������Һ������Ϊ________________________��
���������Ʒ��Fe���ʵ���������__________(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ӻ�ˮ����ȡþ�Ĺ�ҵ������ͼ��ʾ������˵������ȷ����
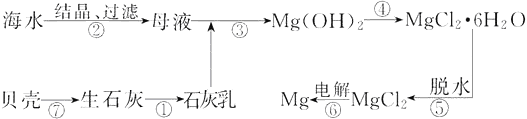
A. �ô˷���ȡþ���ŵ�֮һ��ԭ����Դ�ḻ
B. ��ʵ���ҽ������IJ������õ�����������ǯ�����������ƾ���
C. ���������MgCl2ʱ��������Cl2����ѭ������
D. �������������еķ�Ӧδ�漰�û���Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽ�еķ�Ӧ���»��ߴ���ʾȼ���ȵ���( )
A. NH3(g)��5/4O2(g)===NO(g)��3/2H2O(g) ��H����a_kJ��mol��1
B. C6H12O6(s)��6O2(g)===6CO2(g)��6H2O(l) ��H����b_kJ��mol��1
C. 2CO(g)��O2(g)===2CO2(g) ��H����c_kJ��mol��1
D. CH3CH2OH(l)��1/2O2(g)===CH3CHO(l)��H2O(l) ��H����d_kJ��mol��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com