
��10�֣��±�ΪԪ�����ڱ��ġ����֣������Ԫ�آ�һ���ڱ��е�λ�ã��ش��������⡣
��1������������Ԫ�طǽ�������ǿ��Ԫ�ص�ԭ�ӽṹʾ��ͼ��___________��
��2���ڢۢ�����������Ӧˮ����������ǿ������˳����___________���ѧʽ����
��3���õ���ʽ��ʾ����⻯����γɹ��̣�_________________________________��
��4�����п����жϢݺ͢�����ǿ������______________________������ţ���
A�����ʵ��۵�ݱȢ� B�����ϼۢݱȢ�
C��������ˮ��Ӧ�ݱȢ��� D������������ˮ����ļ��ԢݱȢ�ǿ
��5����������Ԫ�ص����ʼ��������ת����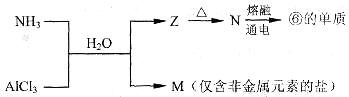
A��M�Ļ�ѧʽ��___________��
B��N���ɢĵ��ʷ�Ӧ�Ļ�ѧ����ʽ��_________________________________��
��1��Cl ����2��HNO3>H2CO3>H2SiO3����3��
����2��HNO3>H2CO3>H2SiO3����3��
��4��C��D����5��A��NH4Cl��B.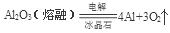
���������������1��ͬһ���ڵ�Ԫ�أ�����ԭ������������Ԫ�صķǽ���������ǿ������������Ԫ�طǽ�������ǿ��Ԫ����Cl,��ԭ�ӽṹʾ��ͼ�� ����2���ڢۢ�����������Ӧˮ����ֱ���H2CO3��HNO3��H2SiO3,������ǿ������˳����HNO3>H2CO3>H2SiO3����3������⻯��HCl���γɹ��̵���ʽ��ʾ�ǣ�
����2���ڢۢ�����������Ӧˮ����ֱ���H2CO3��HNO3��H2SiO3,������ǿ������˳����HNO3>H2CO3>H2SiO3����3������⻯��HCl���γɹ��̵���ʽ��ʾ�ǣ� ����5������Na;����Al��A�����ʵ��۵�Na��Al�۵�ͣ�������������Ե�ǿ���أ�����B��Ԫ�صĽ�����ǿ����Ԫ�صĻ��ϼ۵ĸߵ��أ�����C��Ԫ�صĽ�����Խǿ���䵥����ˮ�����û���������Խ���ף���˵�����ˮ��Ӧ�ݱȢ��ң�����֤�������Ļ�Ե�ǿ������ȷ��D��Ԫ�صĽ�����Խǿ��������������Ӧ��ˮ����ļ��Ծ�Խǿ������������ˮ����ļ��ԢݱȢ�ǿ���ܹ�֤��Ԫ�ؽ����Ե�ǿ������ȷ����5��������ˮ��Һ��AlCl3��Һ������Ӧ����NH4Cl��Al(OH)3��Al(OH)3���ȷֽ����Al2O3��ˮ���������Al2O3����Al��������A��M�Ļ�ѧʽ��NH4Cl��B��N���ɢĵ��ʷ�Ӧ�Ļ�ѧ����ʽ��
����5������Na;����Al��A�����ʵ��۵�Na��Al�۵�ͣ�������������Ե�ǿ���أ�����B��Ԫ�صĽ�����ǿ����Ԫ�صĻ��ϼ۵ĸߵ��أ�����C��Ԫ�صĽ�����Խǿ���䵥����ˮ�����û���������Խ���ף���˵�����ˮ��Ӧ�ݱȢ��ң�����֤�������Ļ�Ե�ǿ������ȷ��D��Ԫ�صĽ�����Խǿ��������������Ӧ��ˮ����ļ��Ծ�Խǿ������������ˮ����ļ��ԢݱȢ�ǿ���ܹ�֤��Ԫ�ؽ����Ե�ǿ������ȷ����5��������ˮ��Һ��AlCl3��Һ������Ӧ����NH4Cl��Al(OH)3��Al(OH)3���ȷֽ����Al2O3��ˮ���������Al2O3����Al��������A��M�Ļ�ѧʽ��NH4Cl��B��N���ɢĵ��ʷ�Ӧ�Ļ�ѧ����ʽ�� ��
��
���㣺����Ԫ�ص��ƶϡ����ĵ���ʽ���ṹʾ��ͼ��ʾ��Ԫ�صĽ����ԵıȽϡ����ʵ��ƶϡ���ѧ����ʽ����д��֪ʶ��


 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪��2FeCl3+Cu=2FeCl2+CuCl2����ʢ���Ȼ�����Һ���ձ���ͬʱ�������ۺ�ͭ�ۣ���Ӧ���������ܳ��ֵĽ����
| A���ձ�����ͭ���� |
| B���ձ���������ͭ |
| C���ձ�������ͭ���� |
| D���ձ�������ͭ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������һ����Ҫ�Ļ���ԭ�ϣ������Ʊ�һϵ�����ʣ���ͼ��ʾ��������˵���������
| A����ʽ������ˮ���ܲ���Fe(OH)3���壬��������ˮ�� |
| B��Ϊ��ֹNH4HCO3�ֽ⣬����FeCO3���ڽϵ��¶��½��� |
| C������KSCN��Һ����(NH4)2Fe(SO4)2�Ƿ����� |
| D�������£�(NH4)2Fe(SO4)2��ˮ�е��ܽ�ȱ�FeSO4�Ĵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�ij���������Al��(NH4)2SO4��MgCl2��AlCl3��FeCl2�е�һ�ֻ�����ɣ���ȡ�ù����������ʵ�飬����������й�����������ʾ(������������ݾ�������ɱ�״����)��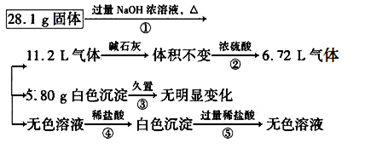
��ش��������⣺
��1����������Ƿ����FeCl2��________(����ڡ������ڡ�)��
��2����������Ƿ����(NH4)2SO4��______(����ڡ������ڡ�)������ж�������____________��
��3��д����Ӧ�ܵ����ӷ���ʽ��____________________��
��4������ݼ����жϻ�������Ƿ����AlCl3��_____(д������ж����ݣ�������д�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�ij�о���ѧϰС��Ϊ�˲ⶨijƷ�ƺϽ������ĺ����������������ʵ�飺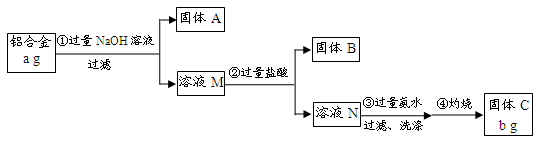
��֪����1�������Ͻ����Ҫ�ɷ�Ϊ����þ��ͭ���衣
��2�������£����ܺ�NaOH������Ӧ��Si+2NaOH+H2O===Na2SiO3+2H2����
��ش��������⣺
��1������A�ijɷ��� ��
��2�����ɹ���B�����ӷ���ʽΪ ��
����������ɳ��������ӷ���ʽΪ ��
��3�����鲽����г����Ƿ�ϴ�Ӹɾ���ʵ�����Ϊ
��
��4������Ʒ���������������� ������a��b��ʾ��
��5�������������������ʹ�ⶨ���ƫ����� ��
| A���ڢٲ��м���NaOH��Һ���� | B���ڢڲ��м��������ʱ |
| C���ڢ۲��г���δ������ˮϴ�� | D���ڢܲ��Գ������ղ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ����Ҫ�IJ��ϣ�����������������벻���������±���ʾ�˽����˳�����ͭ������������Ԫ�ر�������ģ�������õĴ��������
33. ����ͼ�����ݺ��йػ�ѧ֪ʶ������Ϊ�������ģ�������õ��Ⱥ�˳���______�йأ���д��ţ���
a. �����ĵ����� b. ����Ԫ���ڵؿ��еĺ���
c. �����Ļ��˳�� d. ��������չ��
34. ������������ʹ�������������ͽ�������Ҫ��־֮һ�����û�ѧ����ʽ��ʾ��¯������ԭ��___________________________________________��
35. �Ƚ��������Ļ����ԣ�����һ��ʵ����ʵ˵����__________________________________��
�������ڿ�����ȴ���ֳ���ǿ�Ŀ������ԣ���ԭ����____________________________��
36. ��������������������θ�����,Ҳ����ǿ����Һ��Ӧ, д�����������ĵ��뷽��ʽ___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(6��)��ѧ��ѧ����������A��B��C��D��E��X����ͼ��ʾת����ϵ����֪A��D�ǵ��ʣ�BΪ�������Ӧ���ڸ����·�����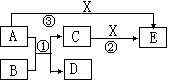
��1����A��D��XΪ�ǽ������ʣ�E�dz������������壬��AΪ____________��XΪ___________���û�ѧʽ��գ���
��2����AΪ�������ʣ�DΪ�ǽ������ʣ�XΪ���ᣬ��Ӧ�ٵĻ�ѧ����ʽΪ________________����Ӧ�ڵ����ӷ���ʽ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʽ������[Fe(OH)SO4]��һ��������ˮ����������Ч����������ҵ�����÷���м�����������������������ȣ�������ʽ�������Ĺ����������£�
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ȼ���������ض��dz�����ˮ����������ͼΪ�Ʊ��Ȼ�������һ�������Ʊ�������صĹ������̡�
��ش��������⣺
��1���Ȼ����ж�����;���������ӷ���ʽ��ʾ������;��ԭ����
���Ȼ�������ˮ��______________________��
����FeCl3��Һ��32%��35%����ʴͭӡˢ��·��____________________________��
��2�����ռ�X�Ļ�ѧʽΪ ���� ��������Y�Ļ�ѧʽΪ________________��
��3�����������·�Ӧ�ٵ����ӷ���ʽΪ____________________________________��
��4�����̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ
2KOH��Na2FeO4��K2FeO4��2NaOH������ݸ��ֽⷴӦԭ��������Ӧ������ԭ��_________��
��5��K2FeO4��ˮ��Һ��������Ӧ��4FeO42-+10H2O 4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
| A��H2O | B��ϡKOH��Һ������� | C��NH4Cl��Һ������� | D��Fe(NO3)3��Һ������� |
 CrO42-��Fe(OH)3����OH-
CrO42-��Fe(OH)3����OH- Cr2O72-��H2O
Cr2O72-��H2O 2Cr3����6Fe3����7H2O
2Cr3����6Fe3����7H2O�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com