
���淴Ӧ2NO2 2NO+O2�����������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
2NO+O2�����������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
�ٵ�λʱ��������n mol O2 ��ͬʱ����2n molNO �ڵ�λʱ��������n mol O2 ��ͬʱ����2n molNO2 ����NO2��NO��O2�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���ʵı�Ϊ2:2:1��״̬ �ܻ���������ɫ���ٸı��״̬ �ݻ��������ܶȲ��ٸı��״̬
A.�٢� ���� B.�ڢ� ������ C.�٢ۢ� ������ D.�٢ڢۢܢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һ���½ṹ���͵Ŀ������ײ����Ϳ����̲������Ļ������ṹ����ͼ��ʾ���й����ص�˵����ȷ���ǣ� ��
A�������к���4������̼ԭ��
B���������Ȼ�����Һ������ɫ��Ӧ
C���ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ
D��1mol����������11molNaOH��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��48 g RO42���У������������������������6.02��1023������Rԭ�ӵ�Ħ������Ϊ________��
����һ���ƿ������ΪM1 g����ƿ���������������ΪM2 g������ͬ״���£����ij�ij����A��������ΪM3 g����A����Է�������Ϊ________��
(2)(2014�콭��ʦ������ĩ)һ������������������ȼ�գ����û������100 mL 3.00 mol��L��1��NaOH��Һ(�ܶ�Ϊ1.12 g��mL��1)ǡ����ȫ���գ������Һ�к���NaClO�����ʵ���Ϊ0.050 0 mol��
��ԭNaOH��Һ����������Ϊ________
��������Һ��Cl�������ʵ���Ϊ________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������繤ҵ���õķ�չ���˿ڵľ�����ȫ����Դ���ż�������������Խ��Խ���ص����⣬��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӡ�
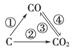
(1)��ͼΪC����������ı仯��ϵͼ�����ٱ仯���û���Ӧ�����仯ѧ����ʽ��Ϊ______________________________________��
ͼ�б仯������Щ�����ȷ�Ӧ________(�����)��
(2)�״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ�Ͽ������·����ϳɼ״���
����һ��CO(g)��2H2(g)CH3OH(g)
��������CO2(g)��3H2(g)CH3OH(g)��H2O(g)
��25�桢101 kPa�£�1�˼״���ȫȼ�շ���22.68 kJ��д���״�ȼ�յ��Ȼ�ѧ����ʽ��_____________________________________________��
ij�������糧CO2������ŷ�����2 200��֣�������CO2��ȫת��Ϊ�״������������ɴ˻�õļ״���ȫȼ�շ���Լ��________kJ(������λ��Ч����)��
(3)������ұ������������һ����Ӧ�ǽ�ԭ�Ͻ��ʯת����TiO2(���ʯ)��2C��2Cl2����,TiCl4��2CO����֪��C(s)��O2(g)===CO2(g)����H����393.5 kJ·mol��1
2CO(g)��O2(g)===2CO2(g)����H����566 kJ·mol��1
TiO2(s)��2Cl2(g)===TiCl4(s)��O2(g)����H����141 kJ·mol��1
��TiO2(s)��2Cl2(g)��2C(s)===TiCl4(s)��2CO(g)�Ħ�H��________��
(4)���������ھ�������������ˮ������������ҵ�������ΪƯ�����������������������ҿ������������е��ʷ�Ӧ���磺
6Ag(s)��O3(g)===3Ag2O(s)����H����235.8 kJ·mol��1��
��֪��2Ag2O(s)===4Ag(s)��O2(g)
��H����62.2 kJ·mol��1��
��O3ת��ΪO2���Ȼ�ѧ����ʽΪ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ݱ�����ȫ��ÿ�귢��������ʴ����ɵ�ֱ�Ӿ�����ʧ����ǧ����Ԫ�����и��缫��Ӧ
ʽ�У��ܱ�ʾ���ĵ绯ѧ��ʴ���� �� ��
�� Fe��2e-=Fe2+ �� 2H++2e-=H2�� �� Fe��3e-=Fe3+
�� 2H2O+O2+4e-=4OH- �� 4OH-��4e-=2H2O+O2��
��A.�٢ڢ� B.�ڢۢ� C.�٢ڢ� D.�٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Cl2����ijЩ�����л���ʱ�����������HCl�����÷�ӦA��4HCl��O2 2Cl2��2H2O����ʵ���ȵ�ѭ�����á�
2Cl2��2H2O����ʵ���ȵ�ѭ�����á�
 ��֪����ӦA�У�4mol HCl���������ų�115.6kJ��������
��֪����ӦA�У�4mol HCl���������ų�115.6kJ��������
�� ��Ͽ�1 mol H—O����Ͽ�1 mol H—Cl�������������ԼΪ ( )
A��16kJ B��24kJ C��32kJ D��48kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ��װ���е缫a��b��Ϊ̼�������ձ�����ʢ��Һ��Ϊ500mL1.0mol/L��
��AΪ (�ԭ��ء����ء�)������Ag�缫�ĵ缫��ӦʽΪ�� ��
���� ��Ӧ(���������ԭ��)��
��Bװ���еĵ缫b��Ϊ �����缫��ӦʽΪ ��
�ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
�ǹ���һ��ʱ���ZnƬ��������6.5gʱ��a���ݳ��������ڱ�״���µ���� L��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��a��b��ʯī�缫��ͨ��һ��ʱ���b��������Һ�Ժ�ɫ������˵����ȷ����
A��X���ǵ�Դ������Y���ǵ�Դ����
B��a���ĵ缫��Ӧ��2Cl����2e��===Cl2��
C����������CuSO4��Һ��pH������
D��Pt������6.4 g Cu����ʱ��b������2.24 L(��״��)����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼��Ƴ����������Ħ������ijͬѧ�����һ���Ʊ�̼��Ƶķ�����������ͼ���£�(����ʯ��ʯ��������SiO2)

�ش��������⣺
(1)�������110��ʯ��ʯ�õ�����66�֡���״�������ɶ�����̼�����Ϊ______________L��ʯ��ʯ��̼��Ƶ���������Ϊ______________%��
(2)����ڢٲ���Ӧ��ȫ���У���ڢڲ���Ӧ���˺�õ��IJ����������ijɷ�Ϊ________________________��
(3)�ڢ۲���Ӧһ�㲻����ͨ��CO2����Ҫԭ����______________________��
��Ӧ�����ӷ���ʽΪ_____________________________________��
(4)CaCO3��һ���������ʣ�25��ʱ��Ksp��2.8��10��9���ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ2.0��10��4 mol/L�������ɳ�������CaCl2��Һ�����ʵ���Ũ����С��______________��
(5)ijѧ����ʯ��ʯΪԭ�ϣ��������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼ���£�
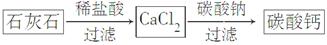
��ǰһ������Ƚϣ��÷������ŵ���_________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com