
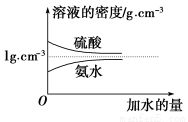
��֪���ᡢ��ˮ���ܶ�������ˮ���Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
| ���ʵ����ʵ���Ũ��/mol��L��1 | ��Һ���ܶ�/g��cm��3 |
���� | c1 | ��1 |
��ˮ | c2 | ��2 |
(1)�����������������Ϊ________(��д��λ���ú�c1����1�Ĵ���ʽ��ʾ)��
(2)���ʵ���Ũ��Ϊc1 mol��L��1��������ˮ��������(��Ϻ���Һ����仯���Բ���)��������Һ�����ʵ���Ũ��Ϊ________mol��L��1��
(3)���ʵ���Ũ��Ϊc2 mol��L��1�İ�ˮ��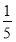 c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(��������������С������������������ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________
c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(��������������С������������������ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________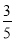 c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��
c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��3-2��ϰ���������棩 ���ͣ������
��0.1 mol��þ�������������100 mL 2 mol/L H2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����ش�
(1)���ڵμ�NaOH��Һ�Ĺ����У���������m�����NaOH��Һ�����V�仯����ͼ��ʾ����V1��160 mLʱ���������ĩ��n(Mg)��________mol��V2��______________mL��
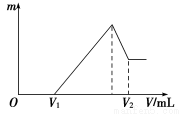
(2)���ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)��____________mL��
(3)���������Ϊ0.1 mol������þ�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���450 mL 1 mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��2-3��ϰ���������棩 ���ͣ�ѡ����
����������ȷ����(����)
A����������ԭ��Ӧ�У��϶���һ��Ԫ�ر���������һ��Ԫ�ر���ԭ
B��Ԫ���ɻ���̬�������̬ʱ�������ܱ�������Ҳ���ܱ���ԭ
C��Ԫ�ص�ԭ�ӵ�ʧ���Ӻ�һ���γ�8���ӵ��ȶ��ṹ
D���е��ʲμӻ����ɵķ�Ӧһ������������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��2-1��ϰ���������棩 ���ͣ������
��������ɸ�������ɺ����ʽ��з��ࣺ
(1)��ͼ��ʾ�����ʷ����������________
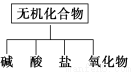
(2)��Na��K��H��O��C��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��ڢۢ��ĺ��档
������� | �� | �� | �� | ������ |
��ѧʽ | HCl��____ | ��___��Ba(OH)2 | ��Na2CO3��____ | ��CO2��Na2O2 |
(3)д����ת��Ϊ���Ļ�ѧ����ʽ_____________________________
(4)���������������ΪO2��Դ�ķ�Ӧԭ��Ϊ��___________________________
(5)ʵ�����Ʊ�������________��________��Ӧ�����������ķ�����____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��2-1��ϰ���������棩 ���ͣ�ѡ����
ij��Ʒ�Ĺ���������������������ǿ������������������й�������
ȷ����(����)
A��������������ͬ���칹�壬���ߵ�ת�����ڻ�ѧ�仯
B������ƽ�����еij������������ߣ����������ϵ�����
C�������ж������Բ�������ˮ�ľ���
D����Ϊ�����ֽ��������������Կ����г����ĺ���Խ��Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��1-3��ϰ���������棩 ���ͣ�ѡ����
��100 mL HNO3��H2SO4�Ļ����Һ�У�����100 mL 0.2 mol/L Ba(OH)2��Һ��pH
��Ϊ7��ͬʱ����2.33 g��ɫ��������ԭ�����Һ��HNO3�����ʵ���Ũ��Ϊ(����)
A��0.1 mol/L B��0.2 mol/L
C��0.3 mol/L D��0.4 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��1-3��ϰ���������棩 ���ͣ�ѡ����
��������Һ��1 L 0.1 mol��L��1NaCl��Һ��ϣ�������Һ��c(Cl��)������(����)
A��50 mL 1 mol��L��1NaCl��Һ
B��20 mL 2 mol��L��1AlCl3��Һ
C��30 mL 1 mol��L��1MgCl2��Һ
D��100 mL 3 mol��L��1NaClO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��1-1��ϰ���������棩 ���ͣ�ʵ����
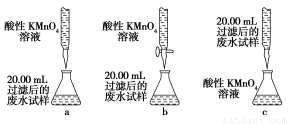
ij��ˮ��Ʒ�к���һ������K����HCO3����SO32�����ס������о�С�����ⶨ����SO32����Ũ�ȡ�
���鷽����
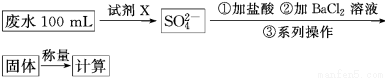
���鷽����
�����ձ�ʢȡ��ˮ����������������̿��ɫ�����ˣ�ȡ��Һ��
����ȷ��ȡ20.00 mL���˺�ķ�ˮ��������0.1 mol��L��1��ɫKMnO4(H2SO4�ữ)��Һ���еζ�(�йط�ӦΪ2MnO4����5SO32����6H��=2Mn2����5SO42����3H2O)��
����¼���ݣ����㡣
(1)���鷽���е����Լ�X����ѡ��________��
a��0.1 mol��L��1 KMnO4(H2SO4�ữ)��Һ
b��0.5 mol��L��1 KOH��Һ
c��������ˮ
d��NaI��Һ
(2)���鷽���У������Լ�X������SO42�������ӷ���ʽΪ________________��
(3)���鷽���У�����������ϵ�в����������IJ�����������Ϊ__________________��
(4)Ϊ���鷽����Ƶ����еζ���ʽ�У����������________(����ĸ���)���ﵽ�ζ��յ�ʱ�ɹ۲쵽��������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ ����ʮ�� ����������ר����ϰ���������棩 ���ͣ�ѡ����
����������һ�ֽ�����ɵĻ�����5.6 g�û�����м���100 gһ����������������ϡ���ᣬ����ǡ����ȫ��Ӧ����������������Ϊm������˵����ȷ���ǣ� ��
A���������ΪFe��Al��m������0.2 g
B���������ΪFe��Zn��m������0.2 g
C���������ΪFe��Zn��m������0.1 g����û������������������Ϊ50%
D���������ΪFe��Al����ϡ������������������һ������7.3%
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com