
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
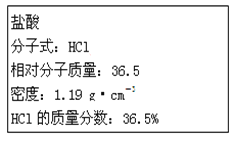
��1����Ũ������HCl�����ʵ���Ũ��Ϊ____mol��L-1��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����___
A.��Һ��HCl�����ʵ��� B.��Һ��Ũ��
C.��Һ���ܶ� D.��Һ��Cl-����Ŀ
��3����������ƿ��ʹ�÷����У�����ȷ����____
A.ʹ������ƿǰ�����Ƿ�©ˮ
B.����ƿ��ˮϴ�������ô�����Һϴ��
C.������Һʱ����������ǹ��壬�ѳƺõĹ�����ֽ��С�ĵ�������ƿ�У�������ˮ����̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
D.������Һʱ����������Һ�壬����Ͳȡ�����ò�����������������ƿ�У�������ˮ����̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
E.�Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
��4��ijѧ����������Ũ���������ˮ����480mL���ʵ���Ũ��Ϊ0.200 mol��L-1��ϡ���ᡣ
�����貣���������ձ�����Ͳ������������ͷ�ι����_____��
�ڸ�ѧ����Ҫ����Ͳ��ȡ___mL����Ũ����������ơ������ƹ����У�����ʵ�������ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�����____
A.ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ���
B.����Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
C.������ƿ�ж���ʱ���ӿ̶���
D.���ݺ������ƿ����ҡ�ȣ�����Һ����ڿ̶��ߣ��ּ�ˮ���̶���
���𰸡�11.9 BC BCD 500mL����ƿ 8.4 C
��������
��1������![]() �����Ũ������HCl�����ʵ���Ũ�ȣ�
�����Ũ������HCl�����ʵ���Ũ�ȣ�
��2������n=cV�жϣ�
��2��A������ƿ��ƿ����ʹ��ǰ����������ƿ�Ƿ�©ˮ��
B������ƿ���ô���Һ��ϴ���������Ƶ���Һ�����ʵ����ʵ���ƫ��
C������ƿ����������һ��Ũ�ȵĶ������������ܹ�������ƿ���ܽ���壻
D��Ӧ�����ձ���ϡ��Ũ��Һ�����ܹ�������ƿ��ֱ��ϡ��
E.Ϊ������Һ��һ���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
��4������480mL���ʵ���Ũ��Ϊ0.200 mol��L-1��ϡ������500mL����ƿ������ϡ��ǰ�����ʵ����ʵ������������Ũ��������������![]() ������
������
��1��![]() mol��L-1��
mol��L-1��
(2)��ҺΪ��һ�ȶ���ɢϵ��Ũ�ȡ��ܶ�����Һ����أ�����n=cV�������ͬʱ�����ʵ����ʵ�����������Ŀ�仯����ѡBC��
��3��A������ƿʹ��ʱ��Ӧ�ȼ���Ƿ�©ˮ��Ȼ��������ˮϴ�Ӹɾ����ɣ���A��ȷ��
B������ƿϴ����������������Һ��ϴ������Ӱ�����Ƶ���Һ��Ũ�ȣ���B����
C������ƿֻ������������Һ������������ƿ���ܽ⣬Ӧ�����ձ����ܽ⣬��C����
D������ƿֻ������������Һ������������ƿ��ϡ�ͣ�Ӧ�����ձ���ϡ�ͣ���D����
E��ҡ��ʱ���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת����E��ȷ��ѡBCD��
��4������480mL���ʵ���Ũ��Ϊ0.200 mol��L-1��ϡ��������500mL����ƿ���ݣ������貣���������ձ�����Ͳ������������ͷ�ι����500mL����ƿ��
����Ҫ11.9mol/L����������ΪV������ϡ�Ͷ����У�0.2mol/L��0.5L=11.9mol/L��V�����V=0.0084L=8.4mL��
A.ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ��ݣ�����ƫ�٣������Ƶ�ϡ��������ʵ���Ũ��ƫС���ʲ�ѡA��
B.����Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬Ũ��������ƫС������ƫ�٣������Ƶ�ϡ��������ʵ���Ũ��ƫС���ʲ�ѡB��
C.������ƿ�ж���ʱ���ӿ̶��ߣ���Һ���ƫС�������Ƶ�ϡ��������ʵ���Ũ��ƫ��ѡC��
D.���ݺ������ƿ����ҡ�ȣ�����Һ����ڿ̶��ߣ��ּ�ˮ���̶��ߣ���Һ���ƫ�������Ƶ�ϡ��������ʵ���Ũ��ƫС���ʲ�ѡD��


 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС������H2C2O4��Һ������KMnO4��Һ��Ӧ��̽������������Ի�ѧ��Ӧ���ʵ�Ӱ������ʵ��ʱ���ȷֱ���ȡ������Һ��Ȼ�����Թ���Ѹ����Ͼ��ȣ���ʼ��ʱ��ͨ���ⶨ��ɫ����ʱ�����жϷ�Ӧ�Ŀ�������С����������·�����
ʵ���� | �����£��Թ��������Լ���������/mL | ��������Һ��ɫ������ɫ����ʱ��/min | |||
0.6mol/L H2C2O4��Һ | H2O | 0.2mol/LKMnO4��Һ | 3mol/L ϡ���� | ||
1 | 3.0 | 2.0 | 2.0 | 3.0 | 4.0 |
2 | 3.0 | 3.0 | 2.0 | 2.0 | 5.2 |
3 | 3.0 | 4.0 | 2.0 | 1.0 | 6.4 |
(1)�����ϱ��е�ʵ�����ݣ����Եõ��Ľ�����______________________________��
(2)��С��ͬѧ���ݾ��������n(Mn2+)��ʱ��仯���Ƶ�ʾ��ͼ����ͼ1��ʾ������ͬѧ�������е�ʵ�����Ϸ��֣���ʵ�������n(Mn2+)��ʱ��仯������Ӧ��ͼ2��ʾ����С��ͬѧ����ͼ2��ʾ��Ϣ������µļ��裬����������ʵ��̽����

��С��ͬѧ����ļ�����___________________________________��
�����������С��ͬѧ���ʵ�鷽��������д���пհס�
ʵ���� | �����£��Թ��������Լ���������/mL | �����Թ��м�����������X | ��������Һ��ɫ������ɫ����ʱ��/min | |||
0.6mol/L H2C2O4��Һ | H2O | 0.2mol/LKMnO4��Һ | 3mol/L ϡ���� | |||
4 | 3.0 | 2.0 | 2.0 | 3.0 | t | |
����X��__________��
������С��ͬѧ����ļ��������ʱ��t__________4.0min����>��=��<����
(3)Ϊ̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬��С��ͬѧ��������ʵ������ϼ�������ʵ�飬���������С��ͬѧ��ɸ�ʵ�鷽�����____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ��װ��ͼ�ش�װ���ô��ű�ʾ��

��ʵ�������ø������������ʱ��Ӧѡ�õķ���װ����_____��������ʱӦѡ�õķ���װ����_____���ƶ�����̼ʱӦѡ�õķ���װ����_____�����������̼�����ѡ��Dװ�ã���װ����ʢ�ŵ��Լ�һ����_______��
����п����Ũ���ᷴӦ�����������Ƴɵ�����ͨ�����������а�ɫ������˵�������к��У�д��ѧʽ��_____����Ӧ����ʽΪ______����Ҫ�Ƴ�������������������װ���Ӧѡ�õ�һ��װ����_____����װ����ҩƷ��������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)ij�л�����C��H��O����Ԫ����ɣ����ģ����ͼ��ʾ��
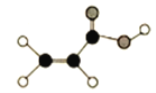
�ٺ��еĹ�����������_______��
��д�����л���������Ʒ�Ӧ�Ļ�ѧ����ʽ_______��
(2)���ֳ����л�����ӵı���ģ��ʾ��ͼ���£����мס��ҡ���Ϊ������Ϊ���������

�ٿ��Լ�����ҵ��Լ�Ϊ_________��
a.ϡ���� b.������Ȼ�̼��Һ c.ˮ d.���Ը��������Һ
�������������ж�����������ζ���Ҳ�����ˮ���ܶȱ�ˮС����_________�������ƣ���
���ҺͶ������ʵ�����1.5mol����ȫȼ����Ҫ�����������ʵ�����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Զ��ijУ�Ļ�ѧ��ȤС�龭����̽��ʵ�飺
(һ)Ϊ��̽��һ�������ܷ�Na2O2��ȫ���գ����������ʵ�顣װ�����£�����װ��ʡ�ԣ���

����������֪����2NO+Na2O2=2NaNO2�������������£�NO��NO2������KMnO4��Һ��Ӧ����NO3-���ش��������⣺
(1)����a���ƣ�____________��
(2)Bƿ��װ�������ǣ�_________��
(3)��NO�ܱ�Na2O2��ȫ���գ�Eװ���е�����Ϊ__________________��
(4)������ƿA�з�Ӧ�Ļ�ѧ����ʽΪ______________________��
(5)Cװ�õ�������_________________________��
(��)ʵ���ҳ���Na2SO3������Ũ���ᷴӦ��ȡSO2
(6)ijͬѧ�ⶨ���ֱ��ʵ�Na2SO3��Ʒ��Na2SO3�ĺ�������֪������������IO3-�ܽ�SO32������ΪSO42-��������ԭΪI-����
���õ�����ƽ��ȡ16.00gNa2SO3�������l00mL��Һ��ȡ25.00mL����ƿ�У������뼸�ε�����Һ��
����0.1000mol/L����KIO3��Һ�������ữ���ζ�������ƽ��ʵ�����ñ�Һ�����ƽ��ֵΪ24.00mL����ζ��յ�ʱ��ƿ�в���������______________��д��������յ������йط�Ӧ�����ӷ���ʽ__________����Ʒ��Na2SO3����������Ϊ_________����������������λ��Ч���֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������к��зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飨����һ�ֲ�����ˮ���ܶȱ�ˮС��Һ�壩��
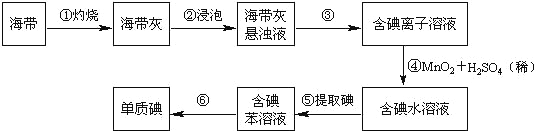
����д���пհף�
��1����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������_______��������������ѡ��������������ñ����ĸ��д�ڿհ״�����
A���ձ� B������ C�������� D�������� E���ƾ��� F��������
��2������۵�ʵ�����������_______�������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ�����������_______��
��3�����������ȡ����Һ��ijѧ��ѡ���ñ�����ȡ���������_______���ڷ�Һ�����У�Ӧ�����Ȼ���ã����ֲ��______�����ţ��������ԡ�
A��ֱ�ӽ����ⱽ��Һ�ӷ�Һ©���Ͽڵ���
B��ֱ�ӽ����ⱽ��Һ�ӷ�Һ©���¿ڷų�
C���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ����ⱽ��Һ���¿ڷų�
D���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ����ⱽ��Һ���Ͽڵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��W������Y��Z��ԭ�������������ӣ�X����������W��Z��������֮�͵�һ�롣m��n��p������ЩԪ����ɵĶ�Ԫ�����r��Ԫ��Y�����嵥�ʣ�nΪ����ɫ��ĩ���������ת����ϵ��ͼ��ʾ�������£�0.01mol/Ls��ҺpHΪ12������˵����ȷ����

A. ԭ�Ӱ뾶��С��W<X<Y B. �����Ӱ뾶��С��Y<Z
C. ���⻯��е�ߵͣ�Z<Y<X D. Y��Z�γɵĻ�����������Ӽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ͷӶ�����������˥�ߣ���������������˥����Ч�á��ϳ�·������

��֪�� ��
��

�ش��������⣺
(1)A�к��������ŵ�����Ϊ___________��
(2)B�Ľṹ��ʽΪ___________��D�Ľṹ��ʽΪ___________��
(3)�ڷ�Ӧ��~���У����ڻ�ԭ��Ӧ����___________��
(4)A��ͬ���칹����ܶ࣬��������������A��ͬ���칹����___________��(�����������칹)��
a.���ڱ�������� b.��ˮ�⣬��ˮ�����֮һ�ܷ���������Ӧ
(5)��Ӧ�ٳ�A��B��Ӧ����C�⣬B��B�����ܷ�����Ӧ������һ�ֻ�����E��д������E�ķ�Ӧ�Ļ�ѧ����ʽ___________��
(6)C�ں˴Ź�����������___________��塣
(7)д���ɼ�ȩ����ȩ�ϳɼ����Ĵ�[C(CH2OH)4]�ĺϳ�·�ߡ�___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����״����V L�����ܽ���1Lˮ�У�ˮ���ܶȽ���Ϊ1g��mL-1����������Һ���ܶ�Ϊ�� g��mL-1����������Ϊ�������ʵ���Ũ��Ϊc mol��L-1�������й�ϵ�в���ȷ���ǣ� ��
A. ��=![]() B. ��=
B. ��=![]()
C. ��=![]() D. c=
D. c=![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com