
ʵ��������480mL0.08mol/LNa2CO3��Һ�ش���������
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ���_______g
��2�����ڳ�����Ʒʱ,ҩƷ������ƽ������,���������ƽ������,��ƽƽ��ʱ,��ʵ�ʳ�����̼���ƾ�����______g(1g����������)
��3��������ƿ����һ�����ʵ���Ũ�ȵ���Һ��������ƿ�����ǣ�����������
����A������ġ���B��ƿ����©ˮ��C���������Ƶ���Һ��ϴ������D���������Ҫ��
��4����ʵ�����������,��Һ��Ũ����ƫ��,ƫ�ͻ��Dz���?
A.��ˮʱԽ���̶���_________
B.���ǽ�ϴ��Һ��������ƿ__________
C.����ƿ�ڱڸ���ˮ���δ���ﴦ��____________
D.�ܽ��û����ȴ����ж���______________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
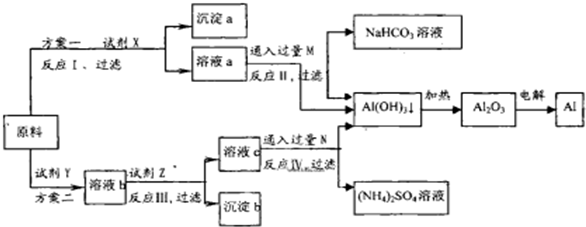
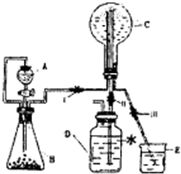
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com