
ΓΨΧβΡΩΓΩ “Έ¬œ¬Θ§œρ10.00mL0.1000molΓΛL-1HClΚΆ0.1000molΓΛL-1CH3COOHΒΡΜλΚœ»ή“Κ÷–ΒΈ»κ0.1000molΓΛL-1NaOH»ή“ΚΘ§»ή“ΚpHΒΡ±δΜ·«ζœΏ»γΆΦΥυ ΨΓΘ“―÷ΣΘΚ≥ΘΈ¬œ¬Θ§Ka(CH3COOH)=1.75ΓΝ10-5ΓΘœ¬Ν––π ω¥μΈσΒΡ «
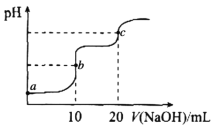
A.aΒψΥυ Ψ»ή“Κ÷–Θ§CH3COOHΒΡΒγάκΕ»‘ΦΈΣ1.75ΓΝ10-2%
B.aΓΔbΓΔc»ΐΒψΥυ Ψ»ή“Κ÷–Θ§Υ°ΒΡΒγάκ≥ΧΕ»Ήν¥σΒΡ «cΒψ
C.cΒψΥυ Ψ»ή“Κ÷–ΘΚc(Na+)>c(C1-)>c(CH3COOH>c(OH-)>c(H+)
D.Φ”»»bΒψΥυ Ψ»ή“ΚΘ§![]() ΒΡ÷ΒΦθ–Γ
ΒΡ÷ΒΦθ–Γ
ΓΨ¥πΑΗΓΩC
ΓΨΫβΈωΓΩ
”…ΧβΗχ–≈œΔΩ…÷ΣΘ§œρ10.00mL0.1000molΓΛL-1HClΚΆ0.1000molΓΛL-1CH3COOHΒΡΜλΚœ»ή“Κ÷–ΒΈ»κ10mL0.1000molΓΛL-1NaOH»ή“ΚΘ§―ΈΥαΗ’ΚΟΆξ»ΪΖ¥”ΠΘ§¥Υ ±»ή“ΚΈΣNaClΚΆCH3COOHΒΡΜλΚœ»ή“ΚΘΜΦ”»κ20mL0.1000molΓΛL-1NaOH»ή“ΚΘ§»ή“Κ÷–ΒΡ»ή÷ ΈΣΘΚNaClΓΔCH3COONaΘ§«“Εΰ’ΏΒΡΈο÷ ΒΡΝΩ≈®Ε»œύΒ»ΓΘΨί¥ΥΫχ––Ζ÷ΈωΓΘ
AΘ°aΒψ»ή“Κ÷–―ΈΥαΕ‘¥ΉΥαΒΡΒγάκ”–“÷÷ΤΉς”ΟΘ§¥Υ ±c(CH3COOH)‘ΦΒ»”Ύc(H+)Θ§‘ρΗυΨίΒγάκΤΫΚβ≥Θ ΐ![]() Θ§Ω…ΒΟ
Θ§Ω…ΒΟ![]() Θ§Ι “―ΒγάκΒΡ
Θ§Ι “―ΒγάκΒΡ![]() Θ§‘ρ¥ΉΥαΒΡΒγάκΕ»=
Θ§‘ρ¥ΉΥαΒΡΒγάκΕ»=![]() Θ§Aœν’ΐ»ΖΘΜ
Θ§Aœν’ΐ»ΖΘΜ
BΘ°aΒψ»ή“ΚΈΣHClΚΆCH3COOHΒΡΜλΚœ»ή“ΚΘ§b»ή“ΚΈΣNaClΚΆCH3COOHΒΡΜλΚœ»ή“ΚΘ§cΒψ»ή“Κ÷–ΒΡ»ή÷ ΈΣΘΚNaClΓΔCH3COONaΘ§‘ΎaΓΔbΒψΥ°ΒΡΒγάκΕΦ ήΒΫ“÷÷ΤΘ§÷Μ”–cΒψΘ§NaClΕ‘Υ°ΒΡΒγάκΈό”ΑœλΘ§CH3COO-ΖΔ…ζΥ°ΫβΘ§¥ΌΫχΝΥΥ°ΒΡΒγάκΘ§Ι aΓΔbΓΔc»ΐΒψΥυ Ψ»ή“Κ÷–Θ§Υ°ΒΡΒγάκ≥ΧΕ»Ήν¥σΒΡ «cΒψΘ§Bœν’ΐ»ΖΘΜ
CΘ°cΒψΥυ Ψ»ή“Κ÷–»ή÷ ΈΣΈο÷ ΒΡΝΩ÷°±»ΈΣ1:1ΒΡNaClΚΆCH3COONaΘ§CH3COO-ΖΔ…ζΈΔ»θΒΡΥ°ΫβΘ§‘ράκΉ”≈®Ε»¥σ–ΓΙΊœΒ”ΠΈΣΘΚc(Na+)>c(C1-)>c(CH3COO-)>c(OH-)> c(CH3COOH)ΘΨc(H+)Θ§Cœν¥μΈσΘΜ
DΘ°bΒψ ±Θ§»ή“ΚΈΣNaClΚΆCH3COOHΒΡΜλΚœ»ή“ΚΘ§“ρΈΣCH3COOHΈΣ»θΒγΫβ÷ Θ§Φ”»»Μα¥ΌΫχCH3COOHΒΡΒγάκΘ§‘ρc(CH3COO-)≈®Ε»‘ω¥σΘ§Εχc(Cl-)≈®Ε»Έό±δΜ·Θ§‘ρ![]() ΒΡ÷ΒΦθ–ΓΘ§Dœν’ΐ»ΖΘΜ
ΒΡ÷ΒΦθ–ΓΘ§Dœν’ΐ»ΖΘΜ
¥πΑΗ―ΓCΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ―–ΨΩΫπ τ”κœθΥαΒΡΖ¥”ΠΘ§ Β―ι»γœ¬ΓΘ
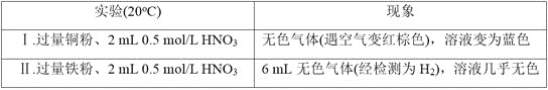
Θ®1Θ©Δώ÷–ΒΡΈό…ΪΤχΧε «_________ΓΘ
Θ®2Θ©Δρ÷–…ζ≥…H2ΒΡάκΉ”ΖΫ≥Χ Ϋ «______________ΓΘ
Θ®3Θ©―–ΨΩΔρ÷–ΒΡ―θΜ·ΦΝ
ΔΌΦΉΆ§―ß»œΈΣΗΟ≈®Ε»ΒΡœθΥα÷–HΘΪΒΡ―θΜ·–‘¥σ”Ύ![]() Θ§Υυ“‘
Θ§Υυ“‘![]() ΟΜ”–ΖΔ…ζΖ¥”ΠΓΘ““Ά§―ß“άΨίΔώΚΆΔρ÷ΛΟςΝΥΦΉΒΡΥΒΖ®≤Μ’ΐ»ΖΘ§Τδ Β―ι÷ΛΨί «____________ΓΘ
ΟΜ”–ΖΔ…ζΖ¥”ΠΓΘ““Ά§―ß“άΨίΔώΚΆΔρ÷ΛΟςΝΥΦΉΒΡΥΒΖ®≤Μ’ΐ»ΖΘ§Τδ Β―ι÷ΛΨί «____________ΓΘ
ΔΎ““Ά§―ßΆ®ΙΐΖ÷ΈωΘ§ΆΤ≤β≥ω![]() “≤Ρή±ΜΜΙ‘≠Θ§“άΨί «_____________Θ§ΫχΕχΥϊΆ®Ιΐ Β―ι÷Λ ΒΗΟ»ή“Κ÷–Κ§”–
“≤Ρή±ΜΜΙ‘≠Θ§“άΨί «_____________Θ§ΫχΕχΥϊΆ®Ιΐ Β―ι÷Λ ΒΗΟ»ή“Κ÷–Κ§”–![]() Θ§Τδ Β―ι≤ΌΉς «____________ΓΘ
Θ§Τδ Β―ι≤ΌΉς «____________ΓΘ
Θ®4Θ©ΗυΨί Β―ιΘ§Ϋπ τ”κœθΥαΖ¥”Π ±Θ§”ΑœλœθΥαΜΙ‘≠≤ζΈο≤ΜΆ§ΒΡ“ρΥΊ”–__________ΘΜ ‘ΆΤ≤βΜΙΩ…Ρή”–ΡΡ–©“ρΥΊ”Αœλ_________Θ®Ν–ΨΌ1ΧθΘ©ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩAΓΔDΓΔFΨυ «≥ΘΦϊ‘ΣΥΊΉι≥…ΒΡΒΞ÷ Θ§B «Β≠ΜΤ…ΪΙΧΧεΘ§≥ΘΈ¬œ¬C «“ΚΧεΓΘ
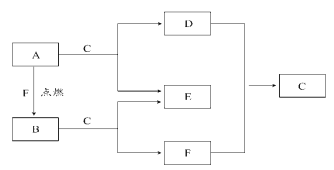
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©BΚΆEΒΡΜ·―ß ΫΘΚB__Θ§E___ΓΘ
Θ®2Θ©A”κCΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ___ΘΜA”κF…ζ≥…BΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___ΓΘ
Θ®3Θ©ΙΊ”ΎAΒΡ–π ω÷–’ΐ»ΖΒΡ «___(Χν–ρΚ≈)ΓΘ
ΔΌA”–Κή«ΩΒΡΜΙ‘≠–‘ ΔΎAΒΡ―φ…ΪΖ¥”Π≥ Ήœ…Ϊ
Δέ…ΌΝΩAΩ…“‘±Θ¥φ‘ΎάδΥ°άο ΔήAΉ≈Μπ ±”Π”ΟœΗ…≥Η«Οπ
Θ®4Θ©ΫΪAΓΔΟΨΓΔ¬ΝΗς0.3molΖ÷±πΖ≈»κ100mL1mol/LΒΡ―ΈΥα÷–Θ§Ά§Έ¬Ά§―Ιœ¬≤ζ…ζΒΡΤχΧεΧεΜΐ÷°±» «__ΓΘ
ΔΌ1ΓΟ2ΓΟ3 ΔΎ6ΓΟ3ΓΟ2 Δέ3ΓΟ1ΓΟ1 Δή1ΓΟ1ΓΟ1
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ±ΫΕΰΒΣΉΩάύ“©ΈοΖζ¬μΈςΡαΘ®FΘ©ΒΡΚœ≥…¬ΖœΏ»γœ¬ΆΦΥυ ΨΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
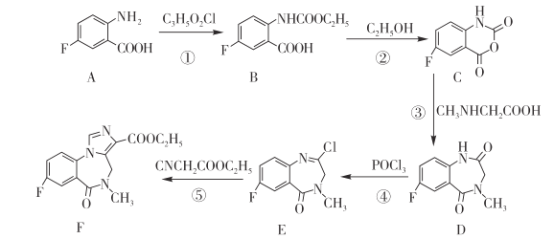
Θ®1Θ©A÷–ΙΌΡήΆ≈”–Ζζ‘≠Ή”ΓΔ_____ΚΆ________ΓΘΘ®ΨυΧνΟϊ≥ΤΘ©
Θ®2Θ©C3H5O2ClΒΡΫαΙΙ ΫΈΣ________ΓΘ
Θ®3Θ©Ζ¥”ΠΔΌΚΆΔΎΒΡΖ¥”Πάύ–ΆœύΆ§Θ§ΤδΖ¥”Πάύ–Ά «___________ΓΘ
Θ®4Θ©Μ·ΚœΈο DΒΡΖ÷Ή” ΫΈΣ___________ΓΘ
Θ®5Θ©Ζ¥”ΠΔί…ζ≥…ΓΑ Έο÷ FΓ± ΚΆ HClΘ§‘ρ EΓζFΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΈΣ________ΓΘ
Θ®6Θ©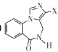 « FΒΡΆ§Ζ÷“λΙΙΧεΘ§Τδ÷– X≤ΩΖ÷Κ§ΓΣCOOH«“ΟΜ”–÷ßΝ¥Θ§¬ζΉψΗΟΧθΦΰΒΡΆ§Ζ÷“λΙΙΧε”–______÷÷Θ®≤ΜΩΦ¬«ΝΔΧε“λΙΙΘ©ΓΘ
« FΒΡΆ§Ζ÷“λΙΙΧεΘ§Τδ÷– X≤ΩΖ÷Κ§ΓΣCOOH«“ΟΜ”–÷ßΝ¥Θ§¬ζΉψΗΟΧθΦΰΒΡΆ§Ζ÷“λΙΙΧε”–______÷÷Θ®≤ΜΩΦ¬«ΝΔΧε“λΙΙΘ©ΓΘ
Θ®7Θ©“―÷ΣΑ±ΜυΥα÷°ΦδΆ―Υ°ΡήΙΜ–Έ≥…Κ§κΡΦϋΒΡΜ·ΚœΈοΘ§«κ…ηΦΤ”…Η Α±ΥαΘ®HOOCCH2NH2Θ©ΚΆCNCH2COOC2H5÷Τ±Η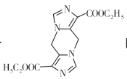 ΒΡΚœ≥…¬ΖœΏ________Θ®ΈόΜζ ‘ΦΝ»Έ―ΓΘ©ΓΘ
ΒΡΚœ≥…¬ΖœΏ________Θ®ΈόΜζ ‘ΦΝ»Έ―ΓΘ©ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A.≥ΘΈ¬œ¬Θ§0.1molΓΛL-1Ρ≥“Μ‘ΣΥα(HA)»ή“Κ÷–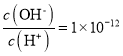 Θ§Φ”»κ…ΌΝΩNaAΨßΧεΜρΦ”Υ°œΓ ΆΘ§»ή“Κ÷–c(OH-)Ψυ‘ω¥σ
Θ§Φ”»κ…ΌΝΩNaAΨßΧεΜρΦ”Υ°œΓ ΆΘ§»ή“Κ÷–c(OH-)Ψυ‘ω¥σ
B.“―÷ΣΘΚ0.1molΓΛL-1KHC2O4»ή“Κ≥ Υα–‘Θ§‘ρ¥φ‘ΎΙΊœΒΘΚc(K+)ΘΨc(HC2O4-)ΘΨc(H2C2O4)ΘΨc(C2O42-)ΘΨc(H+)ΘΨc(OH-)
C.25ΓφΘ§H2SO3![]() HSO3ΓΣ+H+ΒΡΒγάκ≥Θ ΐKa=1ΓΝ10-2molΓΛL-1Θ§ΗΟΈ¬Ε»œ¬NaHSO3Υ°ΫβΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKh=1ΓΝ10-12molΓΛL-1
HSO3ΓΣ+H+ΒΡΒγάκ≥Θ ΐKa=1ΓΝ10-2molΓΛL-1Θ§ΗΟΈ¬Ε»œ¬NaHSO3Υ°ΫβΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKh=1ΓΝ10-12molΓΛL-1
D.0.1molΓΛL-1NaHSO4»ή“Κ÷–ΘΚc(Na+)+c(H+)=c(SO42-)+c(OH-)
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩNOx÷ς“Σά¥‘¥”ΎΤϊ≥ΒΈ≤ΤχΓΘ
“―÷ΣΘΚN2(g)+O2(g)![]() 2NO(g) ΓςH=+180.50kJΓΛmol-1
2NO(g) ΓςH=+180.50kJΓΛmol-1
2CO(g)+O2(g)![]() 2CO2(g) ΓςH=®C566.00kJΓΛmol-1
2CO2(g) ΓςH=®C566.00kJΓΛmol-1
Θ®1Θ©ΈΣΝΥΦθ«α¥σΤχΈέ»ΨΘ§»ΥΟ«…ηœκΧα≥ω‘ΎΤϊ≥ΒΈ≤Τχ≈≈ΤχΙήΩΎΫΪNOΚΆCOΉΣΜ·≥…ΈόΈέ»ΨΤχΧε≤Έ”κ¥σΤχ―≠ΜΖΓΘ–¥≥ωΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΘΚ_______________________________Θ§ΗΟœκΖ®ΡήΖώ Βœ÷______Θ®ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±Θ©ΓΘ
Θ®2Θ©TΓφ ±Θ§ΫΪΒ»Έο÷ ΒΡΝΩΒΡNOΚΆCO≥δ»κ»ίΜΐΈΣ2LΒΡΟή±’»ίΤς÷–Θ§±Θ≥÷Έ¬Ε»ΚΆΧεΜΐ≤Μ±δΘ§Ζ¥”ΠΙΐ≥Χ(0ΓΪ15min)÷–NOΒΡΈο÷ ΒΡΝΩΥφ ±Φδ±δΜ·ΒΡΙΊœΒ»γΆΦΦΉΥυ ΨΓΘ

ΔΌTΓφ ±ΗΟΜ·―ßΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK=_________Θ§ΤΫΚβ ±»τ±Θ≥÷Έ¬Ε»≤Μ±δΘ§‘Όœρ»ίΤς÷–≥δ»κCOΓΔN2Ης0.8molΘ§ΤΫΚβΫΪ_________Θ®ΧνΓΑœρΉσΓ±ΓΑœρ”“Γ±ΜρΓΑ≤ΜΘ©“ΤΕ·ΓΘ
ΔΎΆΦ÷–aΓΔbΖ÷±π±μ Ψ‘Ύ“ΜΕ®Έ¬Ε»œ¬Θ§ Ι”Ο÷ ΝΩœύΆ§ΒΪ±μΟφΜΐ≤ΜΆ§ΒΡ¥ΏΜ·ΦΝ ±Θ§¥οΒΫΤΫΚβΙΐ≥Χ÷–Θ§n(NO)ΒΡ±δΜ·«ζœΏΘ§Τδ÷–±μ Ψ¥ΏΜ·ΦΝ±μΟφΜΐΫœ¥σΒΡ«ζœΏ «________Θ®ΧνΓΑaΓ±ΜρΓΑbΓ±Θ©ΓΘ
Δέ15min ±Θ§»τΗΡ±δΆβΫγΖ¥”ΠΧθΦΰΘ§ΒΦ÷¬n(NO)ΖΔ…ζ»γΆΦΥυ ΨΒΡ±δΜ·Θ§‘ρΗΡ±δΒΡΧθΦΰΩ…Ρή «__________
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΕΰ―θΜ·¬»Θ®ClO2Θ§ΜΤ¬Χ…Ϊ“Ή»ή”ΎΥ°ΒΡΤχΧεΘ© «ΗΏ–ßΓΔΒΆΕΨΒΡœϊΕΨΦΝΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ© Β―ι “”ΟNH4ClΓΔ―ΈΥαΓΔNaClO2Θ®―«¬»ΥαΡΤΘ©ΈΣ‘≠ΝœΘ§Ά®Ιΐ“‘œ¬Ιΐ≥Χ÷Τ±ΗClO2ΘΚ
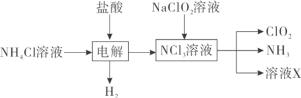
ΔΌΒγΫβ ±―τΦΪΒγΦΪΖ¥”Π ΫΈΣ__________________________ΓΘ
ΔΎ≥ΐ»ΞClO2÷–ΒΡNH3Ω…―Γ”ΟΒΡ ‘ΦΝ «___________Θ®Χν±ξΚ≈Θ©ΓΘ
aΘ°Υ° bΘ°Φν ·Μ“ cΘ°≈®ΝρΥα dΘ°±ΞΚΆ ≥―ΈΥ°
Θ®2Θ©”Ο»γΆΦΉΑ÷ΟΩ…“‘≤βΕ®ΜλΚœΤχ÷–ClO2ΒΡΚ§ΝΩΘΚ
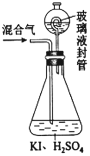
ΔώΘ°‘ΎΉΕ–ΈΤΩ÷–Φ”»κΉψΝΩΒΡΒβΜ·ΦΊΘ§”Ο50mLΥ°»ήΫβΚσΘ§‘ΌΦ”»κ3mLœΓΝρΥαΘΚ
ΔρΘ°‘Ύ≤ΘΝß“ΚΖβΉΑ÷Ο÷–Φ”»κΥ°Θ§ Ι“ΚΟφΟΜΙΐ≤ΘΝß“ΚΖβΙήΒΡΙήΩΎΘΜ
ΔσΘ°ΫΪ“ΜΕ®ΝΩΒΡΜλΚœΤχΧεΆ®»κΉΕ–ΈΤΩ÷–Έϋ ’ΘΜ
ΔτΘ°ΫΪ≤ΘΝß“ΚΖβΉΑ÷Ο÷–ΒΡΥ°ΒΙ»κΉΕ–ΈΤΩ÷–ΘΚ
ΔθΘ°”Ο0.1000molΓΛL-1Νρ¥ζΝρΥαΡΤ±ξΉΦ»ή“ΚΒΈΕ®ΉΕ–ΈΤΩ÷–ΒΡ»ή“ΚΘ®I2+2S2O32-ΘΫ2IΘ≠+S4O62-Θ©Θ§÷Η ΨΦΝœ‘ Ψ÷’Βψ ±Ι≤”Ο»Ξ20.00mLΝρ¥ζΝρΥαΡΤ»ή“ΚΓΘ‘Ύ¥ΥΙΐ≥Χ÷–ΘΚ
ΔΌΉΕ–ΈΤΩΡΎClO2”κΒβΜ·ΦΊΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ______________________ΓΘ
ΔΎ≤ΘΝß“ΚΖβΉΑ÷ΟΒΡΉς”Ο «______________________ΓΘ
ΔέV÷–Φ”»κ÷Η ΨΦΝΘ§ΒΈΕ®÷Ν÷’ΒψΒΡœ÷œσ «______________________ΓΘ
Δή≤βΒΟΜλΚœΤχ÷–ClO2ΒΡ÷ ΝΩΈΣ______gΓΘ
ΔίΡ≥Ά§―ß”ΟΡ≥≤ΩΖ÷ΩΧΕ»ΡΘΚΐ≤Μ«εΒΡ50mLΒΈΕ®ΙήΫχ–– Β―ιΘ§Β±ΒΈΕ®Ιή÷–ΒΡ“ΚΟφ¥Π”Ύ»γΆΦΥυ ΨΒΡΩΧΕ»¥ΠΘ§‘ρΙήΡΎ“ΚΧεΒΡΧεΜΐ________Θ®Χν¥ζΚ≈Θ©ΓΘ
aΘ°Β»”Ύ23.60mL bΘ°Β»”Ύ27.60mL cΘ°–Γ”Ύ23.60mL dΘ°¥σ”Ύ27.60mL
![]()
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–ΈΣΥΡΗω≥Θ”ΟΒΡΒγΜ·―ßΉΑ÷ΟΘ§ΙΊ”ΎΥϋΟ«ΒΡ–π ω’ΐ»ΖΒΡ «
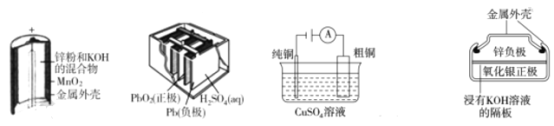
A.ΆΦ(a)÷–Θ§MnO2ΒΡΉς”Ο «¥ΏΜ·ΦΝ
B.ΆΦ(b)Υυ ΨΒγ≥ΊΖ≈ΒγΙΐ≥Χ÷–Θ§ΝΫΦΪΑεΒΡ÷ ΝΩ≤ΜΕœ‘ω¥σ
C.ΆΦ(c)Υυ ΨΉΑ÷ΟΙΛΉςΙΐ≥Χ÷–Θ§ΒγΫβ÷ »ή“Κ÷–Cu2+≈®Ε» Φ÷’≤Μ±δ
D.ΆΦ(d)Υυ ΨΒγ≥Ί≥δΒγΙΐ≥Χ÷–Θ§Ag2O «―θΜ·ΦΝΘ§Βγ≥ΊΙΛΉςΙΐ≥Χ÷–ΜΙ‘≠ΈΣAg
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΗΏ¥ΩΕΰ―θΜ·ΧΦ÷ς“Σ”Ο”Ύ“Ϋ―ß―–ΨΩΦΑΝΌ¥≤’οΕœΦΑΒγΉ”ΙΛ“ΒΘΜΚ§ΧΦΒΡ”–ΜζΈο¥ΦΓΔ»©‘Ύ…ζ≤ζ…ζΜν÷–”–ΙψΖΚ‘Υ”ΟΓΘ
IΘ°(1)ΙΛ“Β…œ”ΟCO2ΚΆH2‘Ύ“ΜΕ®ΧθΦΰœ¬Ζ¥”ΠΩ…Κœ≥…ΕΰΦΉΟ―Θ§“―÷ΣΘΚ
2CO2(g)+6H2(g)ΘΫ2CH3OH(g)+2H2O(g) ΓςH1ΘΫΘ≠107.4kJ/mol
2CH3OH(g)ΘΫCH3OCH3(g)+H2O(g) ΓςH2ΘΫΘ≠23.4kJ/mol
‘ρ2CO2(g)+6H2(g)![]() CH3OCH3(g)ΘΪ3H2O(g) ΓςH3ΘΫ________kJ/mol
CH3OCH3(g)ΘΪ3H2O(g) ΓςH3ΘΫ________kJ/mol
(2)‘Ύ“ΜΕ®ΧθΦΰœ¬ΫΪCO2ΚΆH2≥δ»κ“ΜΙΧΕ®»ίΜΐΒΡΟή±’»ίΤς÷–Θ§‘ΎΝΫ÷÷≤ΜΆ§Έ¬Ε»œ¬ΖΔ…ζΖ¥”ΠΘΚ2CO2(g)+6H2(g)![]() CH3OCH3(g)+3H2O(g)Θ§≤βΒΟCH3OCH3(g)ΒΡΈο÷ ΒΡΝΩΥφ ±ΦδΒΡ±δΜ·»γΆΦΥυ ΨΓΘ
CH3OCH3(g)+3H2O(g)Θ§≤βΒΟCH3OCH3(g)ΒΡΈο÷ ΒΡΝΩΥφ ±ΦδΒΡ±δΜ·»γΆΦΥυ ΨΓΘ
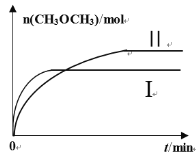
ΔΌ«ζœΏIΓΔΔρΕ‘”ΠΒΡΤΫΚβ≥Θ ΐ¥σ–ΓΙΊœΒΈΣKI_______KΔρ(ΧνΓΑΘΨΓ±ΓΑ=Γ±ΜρΓΑΘΦΓ±)ΓΘ
ΔΎ“ΜΕ®Έ¬Ε»œ¬Θ§œ¬Ν–Ρή≈–ΕœΗΟΖ¥”Π¥οΒΫΜ·―ßΤΫΚβΉ¥Χ§ΒΡ «________(Χν–ρΚ≈)ΓΘ
aΘ°ΜλΚœΤχΧεΟήΕ»≤Μ±δ
bΘ°ΕΰΦΉΟ―ΚΆΥ°’τΤχΒΡΖ¥”ΠΥΌ¬ ÷°±»±Θ≥÷≤Μ±δ
cΘ°v’ΐ(H2)=2vΡφ(H2O)
dΘ°2ΗωC=OΕœΝ―ΒΡΆ§ ±”–3ΗωHΘ≠OΕœΝ―
(3)Κœ≥…ΤχCOΚΆH2‘Ύ“ΜΕ®ΧθΦΰœ¬ΡήΖΔ…ζ»γœ¬Ζ¥”ΠΘΚCO(g)+2H2(g)![]() CH3OH(g) ΓςHΓΘ‘ΎΡ≥―Ι«Ωœ¬Θ§Κœ≥…ΦΉ¥ΦΒΡΖ¥”Π‘Ύ≤ΜΆ§Έ¬Ε»ΓΔ≤ΜΆ§ΆΕΝœ±» ±Θ§COΒΡΉΣΜ·¬ »γΆΦΥυ ΨΓΘ
CH3OH(g) ΓςHΓΘ‘ΎΡ≥―Ι«Ωœ¬Θ§Κœ≥…ΦΉ¥ΦΒΡΖ¥”Π‘Ύ≤ΜΆ§Έ¬Ε»ΓΔ≤ΜΆ§ΆΕΝœ±» ±Θ§COΒΡΉΣΜ·¬ »γΆΦΥυ ΨΓΘ
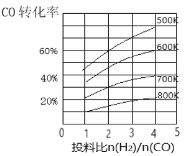
ΔΌ600KΈ¬Ε»œ¬Θ§ΫΪ1molCOΚΆ4molH2≥δ»κ2LΒΡΟή±’»ίΤς÷–Θ§5minΚσΖ¥”Π¥οΒΫΤΫΚβΉ¥Χ§Θ§‘ρ0ΓΪ5minΡΎΒΡΤΫΨυΖ¥”ΠΥΌ¬ v(H2)ΘΫ___________ΓΘ
ΔΎ»τΆΕΝœ±»±Θ≥÷≤Μ±δΘ§…ΐΗΏΈ¬Ε»Θ§ΗΟΖ¥”ΠΤΫΚβœρ_______ΖΫœρ“ΤΕ·(ΧνΓΑ’ΐΖ¥”ΠΓ±ΜρΓΑΡφΖ¥”ΠΓ±)ΓΘ
Δέ…œ ωΚœ≥…ΦΉ¥ΦΒΡΙΐ≥Χ÷–ΧαΗΏCOΒΡΉΣΜ·¬ Ω…≤…»ΓΒΡ¥κ ©”–______ΓΘ(Ν–ΨΌ“Μ÷÷Φ¥Ω…)ΓΘ
ΔρΘ°”ΟΗτΡΛΒγΫβΖ®¥ΠάμΗΏ≈®Ε»““»©ΖœΥ°ΒΡ‘≠άμΈΣΘΚ
Ι”ΟΕη–‘ΒγΦΪΒγΫβΘ§““»©Ζ÷±π‘Ύ“θΓΔ―τΦΪΉΣΜ·ΈΣ““¥ΦΚΆ““ΥαΘ§ΉήΖ¥”ΠΈΣΘΚ2CH3CHO+H2O![]() CH3CH2OH+CH3COOHΓΘ Β―ι “÷–Θ§“‘“ΜΕ®≈®Ε»ΒΡ““»©ΓΣNa2SO4»ή“ΚΈΣΒγΫβ÷ »ή“ΚΘ§ΡΘΡβ““»©ΖœΥ°ΒΡ¥ΠάμΙΐ≥ΧΓΘ
CH3CH2OH+CH3COOHΓΘ Β―ι “÷–Θ§“‘“ΜΕ®≈®Ε»ΒΡ““»©ΓΣNa2SO4»ή“ΚΈΣΒγΫβ÷ »ή“ΚΘ§ΡΘΡβ““»©ΖœΥ°ΒΡ¥ΠάμΙΐ≥ΧΓΘ
ΔΌΒγΫβΙΐ≥Χ÷–Θ§ΝΫΦΪ≥ΐΖ÷±π…ζ≥…““ΥαΚΆ““¥ΦΆβΘ§Ψυ≤ζ…ζΈό…ΪΤχΧεΘ§―τΦΪ≤ζ…ζΤχΧεΒΡΒγΦΪΖ¥”ΠΈΣΘΚ______ΓΘ
ΔΎ‘Ύ ΒΦ ΙΛ“’¥ΠάμΙΐ≥Χ÷–Θ§“θΦΪ«χ““»©ΒΡ»Ξ≥ΐ¬ Ω…¥ο80%ΓΘ»τ‘ΎΝΫΦΪ«χΖ÷±πΉΔ»κ1m3““»©Κ§ΝΩΈΣ400mg/LΒΡΖœΥ°Θ§Ω…ΒΟΒΫ““¥Φ________kg(ΦΤΥψΫαΙϊ±ΘΝτ2ΈΜ–Γ ΐ)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com