
ЁОЬтФПЁПвЛжжИпаЇЮоЛњЫЎДІРэМСЁЊЁЊОлКЯТШЛЏТСОЇЬхЕФЛЏбЇЪНЮЊ[Al2(OH)nCl6ЃnЁЄxH2O]mЁЃЫќПЩЭЈЙ§ЕїНкAlCl3ШмвКЕФpHЃЌДйНјЦфЫЎНтЖјНсОЇЮіГіЁЃЦфжЦБИдСЯжївЊЪЧТСМгЙЄаавЕЕФЗЯдќЁЊЁЊТСЛвЃЌЫќжївЊКЌAl2O3ЁЂAlЃЌЛЙгаSiO2ЕШдгжЪЁЃОлКЯТШЛЏТСЩњВњЕФЙЄвеСїГЬШчЯТЃК
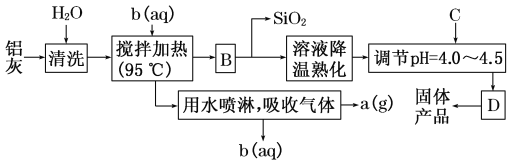
ЃЈ1ЃЉНСАшМгШШВйзїЙ§ГЬжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ_______________________________________ЁЃ
ЃЈ2ЃЉЩњВњЙ§ГЬжаЪЕбщВйзїBЁЂDЕФУћГЦОљЮЊ________ЁЃ
ЃЈ3ЃЉЗДгІжаИБВњЦЗaЪЧ________ЃЌЪдМСbЪЧ________ЁЃ(гУЛЏбЇЪНБэЪО)
ЃЈ4ЃЉЩњВњЙ§ГЬжаПЩбЛЗЪЙгУЕФЮяжЪЪЧ________(гУЛЏбЇЪНБэЪО)ЁЃ
ЃЈ5ЃЉЕїНкpHЃН4.0ЁЋ4.5ЕФФПЕФЪЧ______________________________________________ЁЃ
ЃЈ6ЃЉЮЊЕУЕННЯДПОЛЕФОЇЬхЃЌЩњВњЙ§ГЬжаCЮяжЪПЩбЁгУ________ЁЃ
AЃЎАБЫЎЁЁ BЃЎNaAlO2ЁЁ CЃЎNaOH DЃЎAl2O3ЁЁ EЃЎAl
ЁОД№АИЁПAl2O3ЃЋ6HЃЋ===2Al3ЃЋЃЋ3H2OЁЂ2AlЃЋ6HЃЋ===2Al3ЃЋЃЋ3H2Ёќ Й§ТЫ H2 HCl HCl ДйНјAlCl3ЫЎНтЃЌЪЙОЇЬхЮіГі DE
ЁОНтЮіЁП
ТСЛвжївЊКЌAl2O3ЁЂAlЃЌЛЙгаSiO2ЕШЃЌМгЫЎЧхЯДЕєЦфЫћПЩШмаддгжЪЃЌвђЮЊBКѓЗжРыГіЖўбѕЛЏЙшЃЌЫљвдЭЦГіbШмвКЮЊбЮЫсШмвКЃЌЙ§СПЕФбЮЫсНЋбѕЛЏТСКЭТСЕЅжЪзЊЛЏЮЊТШЛЏТСШмвКЃЌЭЌЪБВњЩњЧтЦјЃЌвђЮЊТШЛЏЧтвзЛгЗЂЃЌЫљвдЧтЦјжаЛьгаТШЛЏЧтЃЌОЫЎХчСмЃЌЕУЕНЦјЬхaЮЊЧтЦјЃЌЭЌЪБЛиЪебЮЫсbЃЛBЮЊТШЛЏТСШмвКжаЛьгаВЛШмадЕФЖўбѕЛЏЙшЃЌЭЈЙ§Й§ТЫЗжРыГіЖўбѕЛЏЙшЃЌТШЛЏТСШмвКОХЈЫѕКѓЕїНкpHЃЌОВжУЁЂЙ§ТЫЕУЕНОлКЯТШЛЏТСОЇЬхЁЃ
ЃЈ1ЃЉгЩЗжЮіПЩжЊbЮЊбЮЫсШмвКЃЌНЋТСКЭбѕЛЏТСзЊЛЏЮЊТШЛЏТСШмвКЃЌЫљвдНСАшМгШШВйзїЙ§ГЬжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊЃКAl2O3ЃЋ6HЃЋ=2Al3ЃЋЃЋ3H2OЁЂ2AlЃЋ6HЃЋ=2Al3ЃЋЃЋ3H2ЁќЃЌЙЪД№АИЮЊЃКAl2O3ЃЋ6HЃЋ=2Al3ЃЋЃЋ3H2OЁЂ2AlЃЋ6HЃЋ=2Al3ЃЋЃЋ3H2ЁќЃЛ
ЃЈ2ЃЉгЩЗжЮіПЩжЊЃЌЩњВњЙ§ГЬжаЪЕбщВйзїBЁЂDЕФУћГЦОљЮЊЙ§ТЫЃЌЙЪД№АИЮЊЃКЙ§ТЫЃЛ
ЃЈ3ЃЉгЩЗжЮіПЩжЊЃЌaЮЊЧтЦјЃЌbЮЊбЮЫсЃЌЙЪД№АИЮЊЃКH2ЃЛHClЃЛ
ЃЈ4ЃЉНСАшМгШШЙ§ГЬжаашвЊМгШыбЮЫсЃЌЮќЪеЦјЬхЙ§ГЬжаПЩЛиЪеЛгЗЂГіЕФHClЃЌЫљвдHClПЩвдбЛЗЪЙгУЃЌЙЪД№АИЮЊЃКHClЃЛ
ЃЈ5ЃЉТШЛЏТСЫЎНтГЪЫсадЃЌЫљвдЕїНкpHЕФФПЕФЪЧЮЊСЫЪЙТШЛЏТСЫЎНтЩњГЩВњЦЗЃЌЙЪД№АИЮЊЃКДйНјAlCl3ЫЎНтЃЌЪЙОЇЬхЮіГіЃЛ
ЃЈ6ЃЉЮЊЕУЕННЯДПОЛЕФОЇЬхЃЌЕїНкpHЕФЪдМСВЛФмв§ШыаТдгжЪЃЌЖјЙ§СПЕФАБЫЎЁЂNaAlO2ЁЂNaOHОљВЛвзГ§ШЅЃЌЛсв§ШыаТдгжЪЃЌ Al2O3ЁЂAlвђЮЊВЛШмгкЫЎЃЌЙ§СПЪБПЩЭЈЙ§Й§ТЫГ§ШЅЃЌЫљвдгУAl2O3ЁЂAlЕїНкpHФмЕУЕННЯДПОЛЕФОЇЬхЃЌзлЩЯЫљЪіЃЌД№АИЮЊDEЁЃ


 НђЧХНЬг§МЦЫуаЁзДдЊЯЕСаД№АИ
НђЧХНЬг§МЦЫуаЁзДдЊЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЪЕбщЯжЯѓгыЪЕбщВйзїВЛЯрЦЅХфЕФЪЧ
ЪЕбщВйзї | ЪЕбщЯжЯѓ | |
A | ЯђЪЂгаИпУЬЫсМиЫсадШмвКЕФЪдЙмжаЭЈШызуСПЕФввЯЉКѓОВжУ | ШмвКЕФзЯЩЋж№НЅЭЪШЅЃЌОВжУКѓШмвКЗжВу |
B | НЋУОЬѕЕуШМКѓбИЫйЩьШыМЏТњCO2ЕФМЏЦјЦП | МЏЦјЦПжаВњЩњХЈбЬВЂгаКкЩЋПХСЃВњЩњ |
C | ЯђЪЂгаБЅКЭСђДњСђЫсФЦШмвКЕФЪдЙмжаЕЮМгЯЁбЮЫс | гаДЬМЄадЦјЮЖЦјЬхВњЩњЃЌШмвКБфЛызЧ |
D | ЯђЪЂгаFeCl3ШмвКЕФЪдЙмжаМгЙ§СПЬњЗлЃЌГфЗжеёЕДКѓМг1ЕЮKSCNШмвК | ЛЦЩЋж№НЅЯћЪЇЃЌМгKSCNКѓШмвКбеЩЋВЛБф |
A. AB. BC. CD. D
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПДгЯТСаЪТЪЕЫљЕУГіЕФЯргІНсТле§ШЗЕФЪЧ
ЪЕбщЪТЪЕ | НсТл | |
A | дкЯрЭЌЮТЖШЯТЃЌЯђ1 mL0.2 mol/LNaOHШмвКжаЕЮШы2ЕЮ0.1 mol/LMgCl2ШмвКЃЌВњЩњАзЩЋГСЕэКѓЃЌдйЕЮМг2ЕЮ0.1 mol/LFeCl3ШмвКЃЌгжЩњГЩКьКжЩЋГСЕэ | ШмНтЖШЃКMg(OH)2>Fe(OH)3 |
B | ФГЦјЬхФмЪЙЪЊШѓЕФРЖЩЋЪЏШяЪджНБфКь | ИУЦјЬхЫЎШмвКвЛЖЈЯдМюад |
C | ЭЌЮТЭЌбЙЯТЃЌЕШЬхЛ§pH=3ЕФHAКЭHBСНжжЫсЗжБ№гкзуСПЕФаПЗДгІЃЌХХЫЎЗЈЪеМЏЦјЬхЃЌHAЗХГіЕФЧтЦјЖрЧвЗДгІЫйТЪПь | HBЕФЫсадБШHAЧП |
D | SiO2МШФмгыЧтЗњЫсЗДгІгжФмгыМюЗДгІ | SiO2ЪЧСНадбѕЛЏЮя |
A.AB.BC.CD.D
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЫљЪОЕФзАжУЃЌCЁЂDЁЂEЁЂFЁЂXЁЂYЖМЪЧЖшадЕчМЋЁЃНЋЕчдДНгЭЈКѓЃЌЯђ(вв)жаЕЮШыЗгЬЊШмвКЃЌдкFМЋИННќЯдКьЩЋЁЃдђЯТСаЫЕЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)
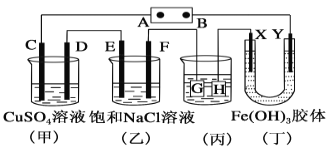
A.ЕчдДBМЋЪЧе§МЋ
B.(Мз)(вв)зАжУЕФCЁЂDЁЂEЁЂFЕчМЋОљгаЕЅжЪЩњГЩЃЌЦфЮяжЪЕФСПжЎБШЮЊ1ЁУ2ЁУ2ЁУ1
C.гћгУ(Бћ)зАжУИјЭЖЦвјЃЌHгІИУЪЧAgЃЌЕчЖЦвКЪЧAgNO3ШмвК
D.зАжУ(ЖЁ)жаYМЋИННќКьКжЩЋБфЩюЃЌЫЕУїЧтбѕЛЏЬњНКСЃДје§ЕчКЩ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгаAЁЂBЁЂCЁЂDЁЂE 5жждЊЫиЃЌЫќУЧЕФКЫЕчКЩЪ§вРДЮдіДѓЃЌЧвЖМаЁгк20ЁЃЦфжаCЁЂEЪЧН№ЪєдЊЫиЃЛAКЭEЪєЭЌвЛзхЃЌЫќУЧдзгЕФзюЭтВуЕчзгХХВМЮЊns1ЁЃBКЭDвВЪєЭЌвЛзхЃЌЫќУЧдзгзюЭтВуЕФpФмМЖЕчзгЪ§ЪЧsФмМЖЕчзгЪ§ЕФСНБЖЃЌCдзгзюЭтВуЩЯЕчзгЪ§ЕШгкDдзгзюЭтВуЩЯЕчзгЪ§ЕФвЛАыЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉгЩетЮхжждЊЫизщГЩЕФвЛжжЛЏКЯЮяЪЧЃЈаДЛЏбЇЪНЃЉ________________________ЁЃ
ЃЈ2ЃЉаДГіCдЊЫиЛљЬЌдзгЕФЕчзгХХВМЪН_________________________ЁЃ
ЃЈ3ЃЉгУЙьЕРБэЪОЪНБэЪОDдЊЫидзгЕФМлЕчзгЙЙаЭ____________________ЁЃ
ЃЈ4ЃЉдЊЫиBгыDЕФЕчИКадЕФДѓаЁЙиЯЕЪЧ___________ЃЌCгыEЕФЕквЛЕчРыФмЕФДѓаЁЙиЯЕЪЧ___________ЁЃЃЈЬюЉЁЂЉЁЂЉЃЌВЂЧвгУдЊЫиЗћКХБэЪОЃЉ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШЁ16.8 gЬМЫсЧтФЦЙЬЬхМгШШвЛЖЮЪБМфКѓЃЌЪЃгрЙЬЬхжЪСП13.7 gЃЌАбЪЃгрЙЬЬхМгШыЕН100 mL2 mol/LЕФЯЁСђЫсжаЃЌГфЗжЗДгІКѓШмвКжаЧтРызгЕФХЈЖШдМЮЊ
A.1.4 mol/LB.3 mol/LC.0.5 mol/LD.2 mol/L
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊБНЛЗЩЯгЩгкШЁДњЛљЕФгАЯьЃЌЪЙЯѕЛљСкЮЛЩЯЕФТБдзгЕФЗДгІЛюаддіЧПЃЌЯжгаФГгаЛњЮяЕФНсЙЙМђЪНШчЯТЃК
1molИУгаЛњЮягызуСПЕФЧтбѕЛЏФЦШмвКЛьКЯВЂЙВШШЃЌГфЗжЗДгІКѓзюЖрПЩЯћКФЧтбѕЛЏФЦЕФЮяжЪЕФСПЮЊa(ВЛПМТЧДМєЧЛљКЭЯѕЛљгыЧтбѕЛЏФЦЕФЗДгІЃЌЯТЭЌ)ЃЌШмвКеєИЩЕУЕНЕФЙЬЬхВњЮядйгызуСПЕФИЩдяМюЪЏЛвЙВШШЃЌгжЯћКФЧтбѕЛЏФЦЕФЮяжЪЕФСПЮЊbЃЌдђaЃЌbЗжБ№ЪЧ( )
вбжЊЃК![]()
AЃЎ5molЃЌ10mol BЃЎ6molЃЌ2mol CЃЎ8molЃЌ4mol DЃЎ8molЃЌ2mol
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПГЃЮТЯТЃЌЯТСаИїзщРызгдкжИЖЈШмвКжавЛЖЈФмДѓСПЙВДцЕФЪЧ
A.ЮоЩЋЭИУїЕФШмвКжаЃКNa+ЁЂMnO4-ЁЂSO32-ЁЂBr-
B.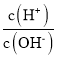 =1ЁС10-12ЕФШмвКжаЃКNa+ЁЂK+ЁЂCO32-ЁЂNO3-
=1ЁС10-12ЕФШмвКжаЃКNa+ЁЂK+ЁЂCO32-ЁЂNO3-
C.ФмЪЙМзЛљГШБфКьЕФШмвКЃКMg2+ЁЂNa+ЁЂHCO3-ЁЂCl-
D.0.1 molЁЄL-1Fe(NO3)2ШмвКЃКH+ЁЂAl3+ЁЂSO42-ЁЂI-
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАДЙйФмЭХЕФВЛЭЌЃЌПЩвдЖдгаЛњЮяНјааЗжРрЃЌЧыжИГіЯТСагаЛњЮяЕФжжРрЃЌЬюдкКсЯпЩЯЁЃ
ЃЈ1ЃЉCH3CH2CH2COOH________ЃЌ
ЃЈ2ЃЉHCOOC2H5________ЃЌ
ЃЈ3ЃЉCH3CH2CH2CH2Cl________ЃЌ
ЃЈ4ЃЉCH3CHO________ЃЌ
ЃЈ5ЃЉ![]() _________ЃЌ
_________ЃЌ
ЃЈ6ЃЉ![]() _________ЃЌ
_________ЃЌ
ЃЈ7ЃЉ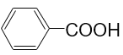 _________ЃЌ
_________ЃЌ
ЃЈ8ЃЉCH3CH2CHЃНCHCH3_________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com