


 2SO3(g)����H=��198 kJ/mol ��2��b
2SO3(g)����H=��198 kJ/mol ��2��b 2SO3(g)����H=��198 kJ/mol��
2SO3(g)����H=��198 kJ/mol��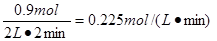 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
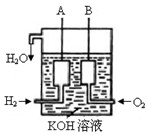
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Q1 = Q2 = Q3 | B��2Q3 = Q1 < Q2�� |
| C��Q3 < Q2 < Q1 | D��Q1 < Q2 < 3Q3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��N2H4+O2=N2+2H2O��H= ��534.4kJ/mol |
| B��N2H4(g)+ O2(g)=N2(g)+2H2O(g)��H = ��16.7kJ/mol |
| C��N2H4(g)+O2(g)=N2(g)+2H2O(l)��H = ��534.4kJ/mol |
| D��N2H4(g)+O2(g)=N2(g)+2H2O(g)��H = ��534.4kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �ų�942kJ�������ɴ��ж�����˵����ȷ���ǣ� ��
�ų�942kJ�������ɴ��ж�����˵����ȷ���ǣ� ��
| A��N4����һ�����͵Ļ����� | B��N4��N2��Ϊͬ�������� |
| C��N4�е��P4�����ף��� | D��1molN4ת��ΪN2���ų�1216kJ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2O(g) = H2(g)��1/2 O2(g)��H��+286kJ��mol-1 |
| B��2H2(g)+ O2(g)=2H2O(l)��H����572kJ��mol-1 |
| C��H2(g)+ 1/2O2(g)= H2O(g)��H����286kJ��mol-1 |
| D��H2(g)��1/2 O2(g)=H2O(l)��H��+286 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
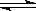 2SO3(g)����H =��Q1kJ/mol������ͬ�¶��£����ܱ�������ͨ��4molSO2��1molO2���ﵽƽ��ʱ�ų�����Q2 kJ�������й�ϵʽ��ȷ����
2SO3(g)����H =��Q1kJ/mol������ͬ�¶��£����ܱ�������ͨ��4molSO2��1molO2���ﵽƽ��ʱ�ų�����Q2 kJ�������й�ϵʽ��ȷ����| A�� Q2=2Q1 | B�� Q2��2Q1 | C�� Q2��Q1 | D��Q2��Q1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ȷ�Ӧ | B�����ȷ�Ӧ | C���ų�832 kJ���� | D������183 kJ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com