
����Ŀ����ҵ�����÷�������(��Ҫ�ɷ�ΪNi��������һ������Zn��Fe��SiO2��CaO��)�Ʊ�������������������£�
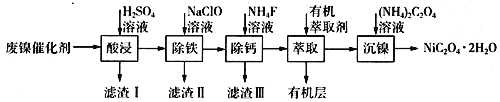
(1)��д��һ������ߡ���������ʵĴ�ʩ��________________________������I�ijɷ���____________(�ѧʽ)��
(2)����ʱ�����Ʋ�ͬ���������Եõ���ͬ������II����֪����II�ijɷ����¶ȡ�pH�Ĺ�ϵ��ͼ��ʾ��
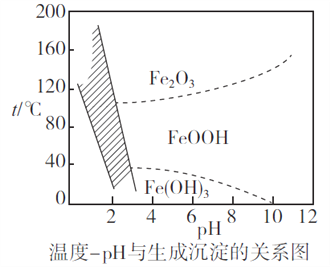
���������¶�40�桢pH=8��������II����Ҫ�ɷ�Ϊ_________________________(�ѧʽ)��
���������¶�80�桢pH=2���ɵõ���������[Na2Fe6(SO4)4(OH)12](ͼ����Ӱ����)��д�����ɻ������Ƶ����ӷ���ʽ��___________________________________________��
(3)��֪����������100 mL��Һ��c(Ca2+)=0.01mol��L-1������100 mL NH4F��Һ��ʹCa2+ǡ�ó�����ȫ����Һ��c(Ca2+)=1��10-5 mol��L-1��������c(NH4F)=_________mol��L-1��[��֪Ksp(CaF2)=5.29��10-9]
(4)�����л���ȡ����������________________________��
(5)ij��ѧ�����Լ��Ļ�ѧʽΪMxNi(SO4)y(MΪ+1�������ӣ�NiΪ+2�ۣ�x��y��Ϊ������)��Ϊ�ⶨ�ö����Լ�����ɣ���������ʵ�飺
I������28.7g�����Լ�������100 mL��ҺA��
��ȷ��ȡ10.00 mL��ҺA����0.40 mol��L-1��EDTA(Na2H2Y)����Һ�ζ����е�Ni2+(���ӷ���ʽΪNi2++H2Y2-=NiY2-+2H+)������EDTA����Һ25.00mL��
����ȡ10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g��
������100 mL�����Լ�ʱ����Ҫ��������ҩ�ס�������ƽ�����������ձ�����Ͳ����ͷ�ι��⣬����Ҫ________________________��
�ڸö����Լ��Ļ�ѧʽΪ________________________________��
���𰸡� �ѷ����������顢�ʵ����ȣ��ʵ��������Ũ�Ȼ����� SiO2��CaSO4 FeOOH 2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+ 6.6��10-2 ��ȥ��Һ�е�Zn2+ 100mL����ƿ (NH4)2Ni(SO4)2
�����������������(1)����Ӱ�췴Ӧ���ʵ����ط�����ߡ���������ʵĴ�ʩ������������SiO2�������Ӧ��CaO�����ᷴӦ�IJ���CaSO4����ˮ��(2)��������II�ijɷ����¶ȡ�pH�Ĺ�ϵͼ����֪�����¶�40�桢pH=8ʱ������II����Ҫ�ɷ�����Na2Fe6(SO4)4(OH)12����Ԫ�ػ��ϼ���+3����֪ClO-��Fe2+����ΪFe3+��ͬʱ����Na2Fe6(SO4)4(OH)12������(3)���ݷ���ʽCa2++2F-= CaF2��������Ca2+����0.002mol NH4F ������Ksp(CaF2)=5.29��10-9������Ca2+����Һ��c(F-)=![]() ��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ������Ҫ��������������Ni2++H2Y2-=NiY2-+2H+����Ni2+�����ʵ���������
��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ������Ҫ��������������Ni2++H2Y2-=NiY2-+2H+����Ni2+�����ʵ���������![]() �ɼ�����Է�������������10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g���ɼ���SO42-�����ʵ���������n(Ni2+)��n(SO42-)����yֵ�����ݻ��ϼ۴����͵��������xֵ����������Է�����������M�����ԭ��������
�ɼ�����Է�������������10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g���ɼ���SO42-�����ʵ���������n(Ni2+)��n(SO42-)����yֵ�����ݻ��ϼ۴����͵��������xֵ����������Է�����������M�����ԭ��������
������(1)����Ӱ�췴Ӧ���ʵ����أ������¶ȡ��ѷ����������顢�ʵ��������Ũ�Ȼ���������������ߡ����������������������SiO2�������Ӧ��CaO�����ᷴӦ�IJ���CaSO4����ˮ����������I�ijɷ���SiO2��CaSO4��(2) �ٸ�������II�ijɷ����¶ȡ�pH�Ĺ�ϵͼ����֪�����¶�40�桢pH=8ʱ������II����Ҫ�ɷ���FeOOH����Na2Fe6(SO4)4(OH)12����Ԫ�ػ��ϼ���+3����֪ClO-��Fe2+����ΪFe3+��ͬʱ����Na2Fe6(SO4)4(OH)12��������Ӧ�����ӷ���ʽ��2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+��(3)���ݷ���ʽCa2++2F-= CaF2��������Ca2+����0.002mol NH4F ������Ksp(CaF2)=5.29��10-9������Ca2+����Һ��c(F-)=![]() �������c(NH4F)=c mol��L-1����
�������c(NH4F)=c mol��L-1����![]() =
=![]() ��c=6.6��10-2��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ��Ҫ100mL����ƿ��������Ni2++H2Y2-=NiY2-+2H+��n(Ni2+)=0.025L
��c=6.6��10-2��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ��Ҫ100mL����ƿ��������Ni2++H2Y2-=NiY2-+2H+��n(Ni2+)=0.025L![]() 0.4 mol��L-1=0.01mol ������
0.4 mol��L-1=0.01mol ������ ![]() ��MxNi(SO4)y����Է�������=
��MxNi(SO4)y����Է�������=![]() �� 10.00 mL��ҺA������������BaCl2��Һ���õ����ᱵ4.66g������SO42-�����ʵ���0.02mol������n(Ni2+)��n(SO42-)=1��y����y=2�����ݻ��ϼ۴����͵�����,x=2���� M�����ԭ��������a����2a+59+96
�� 10.00 mL��ҺA������������BaCl2��Һ���õ����ᱵ4.66g������SO42-�����ʵ���0.02mol������n(Ni2+)��n(SO42-)=1��y����y=2�����ݻ��ϼ۴����͵�����,x=2���� M�����ԭ��������a����2a+59+96![]() =287��a=18������M��NH4+���ö����Լ��Ļ�ѧʽΪ(NH4)2Ni(SO4)2��
=287��a=18������M��NH4+���ö����Լ��Ļ�ѧʽΪ(NH4)2Ni(SO4)2��


 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�á�
�Ʊ�����TiO2�ķ���֮һ��TiCl4ˮ������TiO2xH2O�������ˡ�ˮϴ��ȥ���е�Cl-���ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2��
��������ԭ�ζ����ⶨTiO2������������һ�������£���TiO2�ܽⲢ��ԭΪTi3+������KSCN��Һ��ָʾ������NH4Fe(SO4)2����Һ�ζ�Ti3+��ȫ������Ti4+��
��ش��������⣺
��1��TiCl4ˮ������TiO2xH2O�Ļ�ѧ����ʽΪ______________________________��
��2������TiO2xH2O��Cl-�Ƿ����ķ�����______________________________��
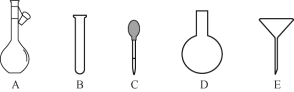
��3������NH4Fe(SO4)2����Һʱ������һ����H2SO4��ԭ����__________��ʹ�õ���������ƽ��ҩ�ס����������ձ�����Ͳ�⣬����Ҫ��ͼ�е�__________������ĸ���ţ���
��4���ζ��յ���ж�������________________________________________��
��5���ζ�����ʱ����ȡTiO2��Ħ������ΪMg/mol������wg������cmol/LNH4Fe(SO4)2 ����ҺVmL����TiO2������������ʽΪ______________________________��
��6���ж����в�����TiO2���������ⶨ�����Ӱ�죨�ƫ�ߡ���ƫ�͡�����Ӱ�족����
���������Ʊ���Һ�����У��ձ��е�NH4Fe(SO4)2��Һ������������ʹ�ⶨ���__________��
�����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬ʹ�ⶨ���__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ���ڱ��в���Ԫ�ص�ij������(Xֵ)��ԭ�������仯�Ĺ�ϵ��

(1)��������ԭ�Ӻ���p����ϵ�������s����ϵ���������ȵ�Ԫ����________(дԪ�ط���)��
(2)ͬ�����ڲ�ͬԪ�ص�Xֵ�仯���ص��� ________________��ͬ�����ڣ�����ԭ������������Xֵ�仯����������________�����ڱ���Xֵ�����ֱ仯�ص�������Ԫ�����ʵ�____________�仯���ɡ�
(3)Xֵ��С��Ԫ�ؼ�����Ԫ�����ڱ���________��
a�����½ǡ� b�����Ͻǡ�����c���ֽ��߸���
(4)���й���Ԫ�ظ����ʵ�˵������ȷ����________(ѡ�����)��
a��Xֵ�ɷ�ӳԪ����������ϼ۵ı仯����
b��Xֵ�ɷ�ӳԭ���ڷ������������ӵ�����
c��Xֵ��С����������Ԫ�ؽ����Ժͷǽ����Ե�ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������װ�ý������ʵ�飬������ȷ���ܴﵽʵ��Ŀ�ĵ���
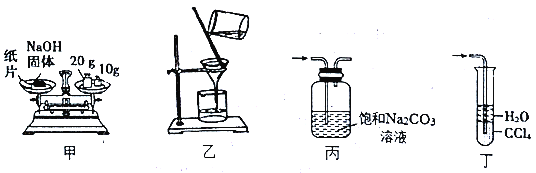
A. ��װ��:��30.0gNaOH���� B. ��װ��:���˳�ȥ��Һ�еIJ���������
C. ��װ��:��ȥCO2�л��е�HCl���� D. ��װ��:����ʵ������NH3��β��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о������仯��������ʣ�������Ч������������滷������Ԫ�ػ��ϼ�һ��������ϵͼ���¡�
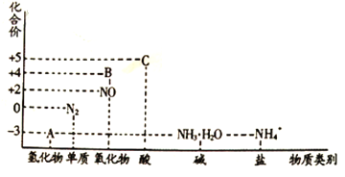
�ش���������:
��1���ڴ����ͼ��ȵ������£�����A����NO�ǹ�ҵ���������Ҫ��Ӧ����ѧ����ʽ��_______________________________________________________________________________��
��2���ڼ��������£�����C��Ũ��Һ��̼���ʷ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ______________��
��3��ʵ�����У�������Һ�к���NH4+�IJ���������______________��
��4������BΪ����ɫ���壬д����������ˮ��Ӧ�����ӷ���ʽ______________________������Ӧ����3.36L(��״��)����Bʱ��ת�Ƶ��ӵ����ʵ���Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ����ͭ��100mLcmol/L��ϡ���ᷴӦ������1.12LNO(��״��)����Ӧ��������Ӧ�����Һ�еμ�1.0mol/L��NaOH��Һ���μӹ����У��������������������NaOH ��Һ�������ϵ����ͼ��ʾ��
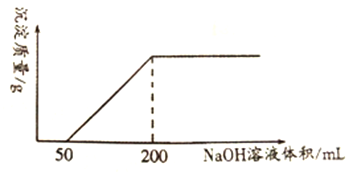
�ش���������:
��1��д��ͭ��ϡ���ᷴӦ�����ӷ���ʽ_____________________________________________��
��2��ͭ��ϡ���ᷴӦ�б���ԭHNO3�����ʵ���Ϊ________mol��
��3��ͭ��ϡ���ᷴӦ�����Һ�У�H+�����ʵ���Ũ��Ϊ________mol/L(��Ӧǰ����Һ����仯���Բ���)��
��4��ϡ��������ʵ���Ũ��c=______mol/L��
��5����ͭ��ϡ���ᷴӦ���ɵ�NOͨ��NaOH ��Һ�У�������20%��H2O2ˮ��Һʹ��ȫ��ת��ΪNaNO3����Ӧ�Ļ�ѧ����ʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)CH��CH3��CH������Ҫ���л���Ӧ�м��壬���ǵĵ���ʽ�ֱ���________��________��________������CH���ĸ�ԭ���ǹ�ƽ��ģ�����������ȣ������Ӧ��________��
(2)�����������ڻ�ѧ��ҵ������ҪӦ�á�N�����������ӣ���д��3��������ԭ�ӹ��ɵĺ�����N�ĵ�������ͬ�����ӵĻ�ѧʽ______��______��______��
(3)N��N���ļ���Ϊ946 kJ/mol��N��N���ļ���Ϊ193 kJ/mol��˵��N2�е�________����________���ȶ�(��ҡ��С�)
(4)CaC2��C![]() ��O
��O![]() ��Ϊ�ȵ����壬O
��Ϊ�ȵ����壬O![]() �ĵ���ʽ�ɱ�ʾΪ________________��1 mol O
�ĵ���ʽ�ɱ�ʾΪ________________��1 mol O![]() �к��еĦм���ĿΪ________��
�к��еĦм���ĿΪ________��
(5)PH3�ڳ�������һ����ɫ���綾������ȼ�����壬���ӽṹ��NH3���ơ��ڳ�����1�����ˮ���ܽ�0.26�����PH3��PH3��±����(HX)����������Ӧ�Ļ�����PH4X��PH4X��ˮ��Һ����ȫˮ��(PH�ṹ������CH4)��PH3�ķ��ӽṹ����״��________����PH��P��H��֮��ļн���_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����д���б���
���� | ������ | ����(g) | ���ʵ���(mol) | Ħ������(g/mol) |
���� | _________ | 14 | _________ | 28 |
ˮ | _________ | _________ | 2 | 18 |
���� | 9.03��1023 | _________ | _________ | 63 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������һ��������ԭ��Ӧ�ķ�Ӧ��������NO��FeSO4��H2O��Fe(NO3)3��HNO3��Fe2(SO4)3. д���÷�Ӧ����ʽ����ƽ���õ����ű������ת�Ʒ�������Ŀ��____________________ ���÷�Ӧ�У���������___________����������Ԫ����___________����������״����11.2L���壬ת�Ƶ�����Ϊ____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com