
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�ء���ش��������⣺
��1����֪����CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H=+49.0kJ/mol
��CH3OH(g)+3/2O2(g)=CO2(g)+2H2O(g) ��H=��192��9kJ/mol
����������ʽ��֪��CH3OH(g)��ȼ����____������ڡ��������ڡ���С�ڡ���192.9kJ/mol����֪ˮ��������Ϊ44 kJ/mol�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ______________________________��
��2�����ݻ�Ϊ2 L���ܱ������У���CO2��H2�ϳɼ״����������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��ע��T1��T2������300 �棩
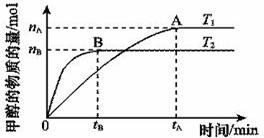
�÷�ӦΪ_________��Ӧ(����ȡ������ȡ�)����T1�¶�ʱ����1 mol CO2��3 mol H2����һ�ܱպ������У���ַ�Ӧ�ﵽƽ�����CO2ת����Ϊa���������ڵ�ѹǿ����ʼѹǿ֮��Ϊ____________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
4���������������Ԫ�ص����λ�������Ԫ��X��ԭ�Ӻ����������M��2����Y��������������ԡ��ش��������⣺
| M | N | ||
| X | Y |
(1)Ԫ��X�����ڱ��е�λ���ǵ�________���ڡ���________�壬�䵥�ʿɲ��õ������________�ķ����Ʊ���
(2)M��N��Y����Ԫ������������Ӧ��ˮ�����У�������ǿ����________��������ǿ����________��(�ѧʽ)
(3)�������(MN)2�ĵ���ʽΪ________��(MN)2��Ϊ��±�أ�������±�����ƣ���������������Һ��Ӧ�Ļ�ѧ����ʽΪ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ����м���泣������(�ɷ�ΪFe2O3)����մ�����ۡ�ijУ�о���ѧϰС�����ʵ�鷽���������¢١��ܲ����÷���м�Ʊ��̷�(FeSO4��7H2O)���塣
����м ��м
��м
 ��Һ(����������м)
��Һ(����������м)
 ��Һ
��Һ �̷�����
�̷�����
���ݸ�ʵ�鷽�����ش��������⣺
(1)������з���м��NaOH��Һ���ȵ�Ŀ����________________________________________________��
(2)��������ձ��ײ�����������м��������________________________________________________��
˵��ʣ����м���õ����ӷ���ʽΪ______________________________________________��
(3)�ڲ�����У���С�������ͼ��������(��ѹ����)װ�ô�����ͨ©������Ŀ����________��________��

(4)��鲽��۵���Һ��û��Fe3���ķ�����_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��ѧ�кܶࡰ���ɡ����������÷�Χ�����и����йء����ɡ��Ƴ��Ľ��ۺ�������(����)
A��Na2O��Na2O2���Ԫ����ͬ���Ƴ���ˮ��Ӧ����Ҳ��ȫ��ͬ
B��SO2��ʪ���Cl2����Ư���ԣ��Ƴ�����Ϻ�Ư���Ը�ǿ
C��H2CO3�����Ա�HClOǿ���Ƴ�CO2ͨ��NaClO��Һ��������HClO
D�����ݳ�����ͭ��Ũ���������ȡNO2���Ƴ�����������Ũ����Ҳ������ȡNO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и����ֵΪ1��2���ǣ� ��
A��0.1 mol/L��0.2 mol/L������Һ��c(H+)֮��
B��0.1 mol/L H2S��Һ��c(S2��)��c(H+)֮��
C��pH=10��Ba(OH)2��Һ��pH=10�İ�ˮ�����ʵ����ʵ���Ũ��֮��
D��pH=3��������pH=3�Ĵ�����Һ��c(SO42��)��c(CH3COO��)֮��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л������У����зǼ��Թ��ۼ������ӻ�������
A��CaC2���������������� B��N2H4
C��Na2S2 D��NH4NO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ���ʼ��仯�����ںܶ���������Ҫ��;�������ͭ����������ߵ��£���ˮ����ͭ������ɱ������
(1)Cuλ��Ԫ�����ڱ��ڢ�B�塣Cu2���ĺ�������Ų�ʽΪ________________________��
(2)��ͼ��ͭ��ij��������ľ����ṹʾ��ͼ����ȷ���þ����������ӵĸ���Ϊ________��
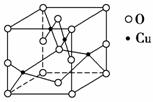
(3)����CuSO4·5H2O��д��[Cu(H2O)4]SO4·H2O����ṹʾ��ͼ���£�
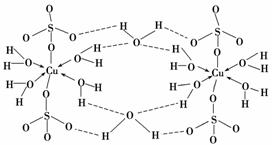
����˵����ȷ����________(����ĸ)��
A���������ṹʾ��ͼ�У�������ԭ�Ӷ�����sp3�ӻ�
B���������ṹʾ��ͼ�У�������λ�������ۼ������Ӽ�
C�������Ƿ��Ӿ��壬���Ӽ�������
D�������е�ˮ�ڲ�ͬ�¶��»�ֲ�ʧȥ
(4)������ͭ��Һ�м��������ˮ��������[Cu(NH3)4]2�������ӡ���֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2���γ������ӣ���ԭ����____________________________��
(5)Cu2O���۵��Cu2S��________(��ߡ��͡�)�������ԭ��_______________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Һ����ʾ���Ĺ�����ʹ�õĻ�ѧ��ϴ��NF3��һ���������壬��洢������������CO2��12 000��20 000�����ڴ����е������ɳ���740��֮�ã������Ǽ��ֻ�ѧ���ļ��ܣ�
| N��N | F��F | N��F | |
| 1 mol�����еĻ�ѧ������ ʱ��Ҫ���յ�����/kJ | 941.7 | 154.8 | 283.0 |
����˵������ȷ����
A������N2(g)�D��2N(g)�ų�����
B������N(g)��3F(g)�D��NF3(g)�ų�����
C����ӦN2(g)��3F2(g)�D��2NF3(g)�Ħ�H��0
D��NF3�������������û�л�ѧ���Ķ��������ɣ��Կ��ܷ�����ѧ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���A�Ǻϳɶ��⾣����������Ҫԭ�ϣ���ṹ��ʽΪ �����м���A�й����ŵ��Լ���˳����ȷ����(����)
�����м���A�й����ŵ��Լ���˳����ȷ����(����)
A���ȼ����Ը��������Һ�����������Һ����
B���ȼ���ˮ��������Ը��������Һ
C���ȼ�������Һ���ȣ��ټ�����ˮ
D���ȼ�������������ͭ���ȣ��ữ���ټ���ˮ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com