
����Ŀ��������������A��B��C��D��E�����ǵ������ӿ�����Na����NH4+��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����NO3-��SO42-��CO32-����֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba��NO3��2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش��������⣺
��1���������У�һ��û�е���������____________��������������ͬ�������εĻ�ѧʽ��_______________________ ��
��2��A�Ļ�ѧʽΪ_____________________��D�Ļ�ѧʽΪ_____________________��D��Һ�Լ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ����__________________________��
��3��E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��________________________________��E��D��Ӧ�����ӷ���ʽ��__________��
��4�����ʵ�����B�������������ӣ�_____________________________________________ ��
���𰸡�Cu2+��Fe3+ ( NH4) 2SO4��Al2(SO4) 3 BaCl2 Na2CO3 CO32����H2O![]() HCO3����OH�� Al3+��3NH3��H2O=Al(OH) 3����3NH4+ 2Al3+��3CO32����3H2O= 2Al(OH)3 + 3CO2�� ȡ����B���Թ��У��μ���������������Һ�����Թ��и�����һ��ʪ��ĺ�ɫʯ����ֽ�����Թܼ��ȣ�����ֽ������˵��B�к���NH4+��
HCO3����OH�� Al3+��3NH3��H2O=Al(OH) 3����3NH4+ 2Al3+��3CO32����3H2O= 2Al(OH)3 + 3CO2�� ȡ����B���Թ��У��μ���������������Һ�����Թ��и�����һ��ʪ��ĺ�ɫʯ����ֽ�����Թܼ��ȣ�����ֽ������˵��B�к���NH4+��
��������
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ����û��Cu2+��Fe3+����D����ɫ��Ӧ�ʻ�ɫ����D����Na+����A����Һ�����ԣ�B��C��E����Һ����������NH4+��Al3+��Ag+��D����Һ�ʼ�����D�к���CO32-�����������ӿ�֪DΪNa2CO3���������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��A��C����Һ��������������A��C��û��SO42-��CO32-���������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��˵��C�к�Ag+����E����Al3+��Ag+��Cl-��SO42-��CO32-���ܹ��棬����C��ΪAgNO3���ް�A��Һ�����ԣ�A�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�������AΪBaCl2�������Ϸ�����֪E�к���Al3+��B�к���NH4+����BaCl2�������ɲ�����ϡ����ij�������B��E�к���SO42-������B��EΪ(NH4)2SO4��Al2(SO4)3��
��1���������У�һ��û�е���������Cu2+��Fe3+��������������ͬ�������εĻ�ѧʽ��(NH4)2SO4��Al2(SO4)3��
��2��A�Ļ�ѧʽΪBaCl2��D�Ļ�ѧʽΪNa2CO3��Na2CO3��Һ�Լ��Ե�ԭ����CO32-+H2OHCO3-+OH-��
��3��Al2(SO4)3�Ͱ�ˮ��Ӧ�����ӷ���ʽ��Al3+��3NH3��H2O=Al(OH) 3����3NH4+��Al2(SO4)3��Na2CO3��Ӧ�����ӷ���ʽΪ2Al3+��3CO32����3H2O= 2Al(OH)3 + 3CO2����
��4������(NH4)2SO4�������������ӵķ���Ϊ��ȡ����B���Թ��У��μ�����NaOH��Һ�����Թܿڸ�����һ��ʪ��ĺ�ɫʯ����ֽ�����ȣ�����ֽ������˵��B��������ΪNH4+��


 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ����
A. FeCl3��Һ�еμӹ���Na2S��Һ��2Fe3++S2- ��2Fe2++S��
B. ��Na2O2����Ͷ��H218O�У�2Na2O2��2H218O ��4Na+��4OH����18O2��
C. ��NH4Al(SO4)2��Һ�е���Ba(OH)2��Һ��ǡ��ʹSO42-��ȫ������NH4+��Al3+��2SO42-��2Ba2+��4OH- �� Al(OH)3����NH3��H2O��2BaSO4��
D. ����������Ũ���ᷴӦ��MnO2+4H++4Cl- ![]() Mn2++2H2O+2Cl2��
Mn2++2H2O+2Cl2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA���������ӵ�����������˵����ȷ���ǣ� ��
A.��״���£�22.4 L CO��CO2�Ļ��������������̼ԭ����һ����NA
B.��״���£�2.24L��������ˮ������Ӧ��ת�Ƶĵ�����ĿΪ0.1 NA
C.���ʵ���Ũ��Ϊ2mol/L��BaCl2��Һ�У�����Cl-����Ϊ4NA
D.��״���£�11.2L H2O����0.5NA����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�����л�������AΪԭ���Ʊ��߾���X��Y�ĺϳ�·����ͼ:
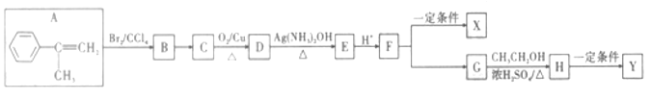
�ش���������:
��1��A�Ļ�ѧ������___________��X�Ľṹ��ʽΪ___________________��
��2��H������������������_____________________��
��3��B��C�ķ�Ӧ������_________��F��G�ķ�Ӧ������________________��
��4��D��E�Ļ�ѧ����ʽΪ__________________��
��5��WΪG��ͬ���칹�壬��������������W��ͬ���칹�干��_________�֡�(�����������칹)��
���ܷ���������Ӧ
����FeCl3��Һ����ɫ
����ʹ������Ȼ�̼��Һ��ɫ
���к˴Ź�������6��壬�ҷ����֮��Ϊ1:1:1:1:2:2�Ľṹ��ʽΪ_____________��
��6����֪: 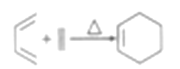
�����![]() ��
��![]() Ϊԭ����
Ϊԭ����![]() �ĺϳ�·������ͼ(�����Լ���ѡ���ϳ�·������ͼʾ�����������)______________
�ĺϳ�·������ͼ(�����Լ���ѡ���ϳ�·������ͼʾ�����������)______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA���������ӵ�����������������ȷ����
A. 14 g��ϩ���ϩ�Ļ��������������ԭ����ĿΪ2NA �� 18 g H2O��18 g D2O������������Ϊ10NA
B. 56 g��ƬͶ������ŨH2SO4������NA��SO2���ӣ��ܱ�������2 mol NO��1 mol O2��ַ�Ӧ������ķ�����Ϊ2NA
C. 1 mol Na������O2��Ӧ������Na2O��Na2O2�Ļ���ת�Ƶĵ�����ΪNA ��0.1molCl2��������ˮ�У�ת�Ƶĵ�����С��0.1NA
D. 25 ��ʱ��pH��13��1.0 L Ba(OH)2��Һ�к��е�OH����ĿΪ0.2NA ��1 L 0.1 mol��L��1��Na2CO3��Һ��������ԭ����ĿΪ0.3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(��Ҫ�ɷ���Al2O3����SiO2��Fe2O3��MgO������)����ȡ�����������ֹ����������£�
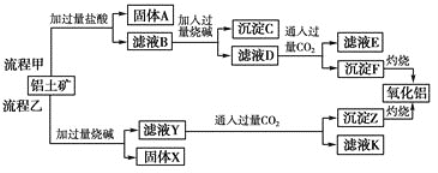
��ش��������⣺
(1) �����Ҽ����ռ������SiO32�������ӷ���ʽΪ________________________________��
(2) д������ҺD����Al(OH)3�����ӷ���ʽ�� ________________________________��
(3)����ҺY�м���NaHCO3��Һ����Һ��pH________(��������������������������С��)��
(4) ��ҺE��K�����ʵ���Ҫ�ɷ���________(�ѧʽ)��
(5) ��֪298 Kʱ��Mg(OH)2���ܶȻ�����Ksp��5.6��10��12��ȡ��������ҺB������һ�������ռ����ﵽ�����ܽ�ƽ�⣬���pH��13.00������¶��²�������Һ�е�c(Mg2��)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ��ijɷ���Cu2O����ͭ��ijɷ���Cu2S������ͭ�����ͭ���ϼ��������·�Ӧ��2Cu2O��Cu2S![]() 6Cu��SO2�������ڸ÷�Ӧ������˵����ȷ����
6Cu��SO2�������ڸ÷�Ӧ������˵����ȷ����
A. �÷�Ӧ��������ֻ��Cu2O B. Cu��������������ǻ�ԭ����
C. Cu2S�������������ǻ�ԭ�� D. ��ԭ������������������ʵ���֮��Ϊ1��6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ�ؼס��ҡ���������ԭ�Ӱ뾶�����������⻯���мס��ҡ��������Ļ��ϼ������ʾ������˵����ȷ����
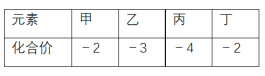
A. �⻯��ķе㣺��>��B. Ԫ�طǽ����ԣ���<��
C. ����������ԣ��� <��D. ����������Ϊ���ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��O2��O3��N2��N4�����͵�Ԫ�صļ��ֵ��ʡ��ش��������⣺
��1��Oԭ���м۵���ռ�ݵĹ����ĿΪ______________��
��2����һ������I1��N__________O(���������)���ڶ�������I2��O����N��ԭ����_________________��
��3��O3�Ŀռ乹��Ϊ__________________�������д��ڴ����������÷�����![]() ��ʾ������m��ʾ�γɵĴ�������ԭ������n��ʾ�γɵĴ������ĵ���������O3�д�����Ӧ��ʾΪ___________________________________��
��ʾ������m��ʾ�γɵĴ�������ԭ������n��ʾ�γɵĴ������ĵ���������O3�д�����Ӧ��ʾΪ___________________________________��
��4��NԪ�صļ���̬�⻯��NH3��H2O���ܽ�Ⱥܴ���ԭ��֮һ��NH3��H2O�����γɷ��Ӽ���������ڰ�ˮ��������Ա�ʾΪH3N��H��N��____________________��__________________________(��д���ּ���)��
��5����֪�������м��ܺͼ�����Ŀ��
��ѧ�� | ����/pm | ����/(kJ��mol��1) |
N-N | 145 | 193 |
N=N | 125 | 418 |
N��N | 110 | 946 |
N2��N4����NԪ�صĵ��ʣ�����N4���������幹�ͣ�Nԭ��ռ����������ĸ����㣬�Ӽ������Ƕȷ���N4�����ȶ���ԶС��N2ԭ����________________________��
��6��Na2O�ľ����ṹ��ͼ��ʾ ��X��ʾO2����Y��ʾNa������O2������λ��Ϊ____________���þ�����ԭ�ӿռ�������Ϊ_____________��[��֪�þ������ⳤΪa pm��r(Na��)��x pm��r(O2��)��y pm]
��X��ʾO2����Y��ʾNa������O2������λ��Ϊ____________���þ�����ԭ�ӿռ�������Ϊ_____________��[��֪�þ������ⳤΪa pm��r(Na��)��x pm��r(O2��)��y pm]
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com