
ΓΨΧβΡΩΓΩ“‘ΦΉ»©ΚΆ±ΫΖ”ΈΣ÷ς“Σ‘≠ΝœΘ§Ψ≠œ¬Ν–ΉΣΜ·Ω…Κœ≥…Ζ”»© ς÷§ΚΆ÷Ί“ΣΒΡ”–ΜζΚœ≥…÷–ΦδΧεD(≤ΩΖ÷Ζ¥”ΠΧθΦΰΚΆ≤ζΈο“―¬‘»Ξ)

“―÷ΣΘΚR1CH2COOCH2+R2COOCH3![]() CH3OH+
CH3OH+
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)Ζ¥”ΠΔΌΒΡΖ¥”Πάύ–ΆΈΣ___________ΘΜAΒΡΜ·―ßΟϊ≥ΤΈΣ___________ΓΘ
(2)C÷–ΥυΚ§ΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣ___________ΘΜDΒΡΖ÷Ή” ΫΈΣ___________ΓΘ
(3)Ζ¥”ΠΔΎΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______________________ΓΘ
(4)AΒΡœ¬Ν––‘÷ ÷–Θ§ΡήΖ¥”≥÷ßΝ¥Ε‘±ΫΜΖΫαΙΙ≤ζ…ζ”ΑœλΒΡ «___________(ΧνΉ÷ΡΗ)ΓΘ
aΘ°Ρή”κ«β―θΜ·ΡΤ»ή“ΚΖ¥”Π
bΘ°Ρή ΙΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΆ …Ϊ
cΘ°œρœΓ»ή“Κ÷–Φ”»κ≈®δεΥ°ΚσΘ§≤ζ…ζ≥ΝΒμ
(5)ΖΦœψΜ·ΚœΈοE(C8H10O2)”κBΒΡΥ°Ϋβ≤ζΈοΜΞΈΣΆ§Ζ÷“λΙΙΧεΘ§1molEΩ…”κ2 mol NaOHΖ¥”ΠΘ§ΤδΚΥ¥≈Ι≤’ώ«βΤΉ÷–”–3ΉιΖε«“ΖεΟφΜΐ÷°±»ΈΣ3ΘΚ1ΘΚ1Θ§‘ρE”–___________÷÷Ω…ΡήΒΡΫαΙΙ(≤ΜΩΦ¬«ΝΔΧε“λΙΙ)ΓΘ
(6)“‘CH3OHΚΆCH3ONaΈΣ‘≠Νœ(ΤδΥϊ ‘ΦΝ»Έ―Γ)Θ§…ηΦΤ÷Τ±ΗCH3COCH2 COOCH3ΒΡΚœ≥…¬ΖœΏΘΚCH3OH![]() ______ΓΘ
______ΓΘ
ΓΨ¥πΑΗΓΩΦ”≥…Ζ¥”Π ΝΎτ«ΦΉΜυ±ΫΖ” Ο―ΦϋΚΆθΞΜυ C19H20O5 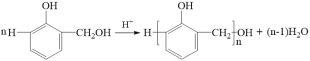 c 4 CH3OH
c 4 CH3OH![]() CH3Cl
CH3Cl![]() CH3CN
CH3CN![]() CH3COOCH3
CH3COOCH3![]() CH3COCH2COOCH3
CH3COCH2COOCH3
ΓΨΫβΈωΓΩ
Θ®1Θ©Ζ¥”ΠΔΌ÷Η‘ΎΥα¥ΏΜ·œ¬Θ§Β»Έο÷ ΒΡΝΩΒΡ±ΫΖ””κΦΉ»©Ζ¥”ΠΘ§±ΫΖ”ΝΎΕ‘ΈΜΒΡ«β‘≠Ή””κΦΉ»©ΒΡτ ΜυΦ”≥……ζ≥…τ«ΦΉΜυ±ΫΖ”Θ§“ρΕχΖ¥”Πάύ–Ά «Φ”≥…Ζ¥”ΠΘ§≤ζΈοAΒΡΟϊ≥Τ «ΝΎτ«ΦΉΜυ±ΫΖ”ΓΘ
Θ®2Θ©C «Ε‘ΦΉ―θΜυ±Ϋ““ΥαΦΉθΞΘ§ΤδΫαΙΙΦρ ΫΈΣ![]() Θ§Ω…÷ΣCΚ§”–Ο―ΦϋΚΆθΞΜυΘ§DΖ÷Ή” ΫΈΣC19H20O5ΓΘ
Θ§Ω…÷ΣCΚ§”–Ο―ΦϋΚΆθΞΜυΘ§DΖ÷Ή” ΫΈΣC19H20O5ΓΘ
Θ®3Θ©Ζ¥”ΠΔΎ «ΝΎτ«ΦΉΜυ±ΫΖ”÷°ΦδœύΜΞΆ―Υ°ΥθΚœ≥…œΏ–ΆΫαΙΙΗΏΖ÷Ή”Θ§ΤδΜ·―ßΖΫ≥Χ ΫΘΚ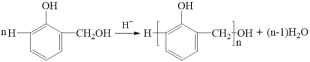 ΓΘ
ΓΘ
Θ®4Θ©A «±ΫΖ”Θ§≤ύΝ¥τ«ΜυΜαΜνΜ·±ΫΜΖ«β‘≠Ή”Θ§ ΙΒΟ“ΉΖΔ…ζΝΎΈΜΚΆΕ‘ΈΜΒΡ»Γ¥ζΖ¥”ΠΘ§άΐ»γ±ΫΖ”ΚΆδεΥ°Ζ¥”Π…ζ≥…2,4,6-»ΐδε±ΫΖ”Θ§Ι ¥πΑΗ―ΓcΓΘ
Θ®5Θ©ΗυΨίEΒΡΖ÷Ή” ΫC8H10O2Ω…Υψ≥ω≤Μ±ΞΚΆΕ»ΈΣ4Θ§ΫαΚœE «ΖΦœψΉεΜ·ΚœΈοΘ§ΥΒΟςΚ§”–±ΫΜΖΘ§≤ύΝ¥Έό≤Μ±ΞΚΆΦϋΜρ’ΏΜΖΘ§”÷1molEΩ…”κ2 mol NaOHΖ¥”ΠΘ§ΥΒΟς±ΫΜΖΝ§Ή≈ΝΫΗωτ«ΜυΘ§ΉνΚσΤδΚΥ¥≈Ι≤’ώ«βΤΉ÷–”–3ΉιΖε«“ΖεΟφΜΐ÷°±»ΈΣ3ΘΚ1ΘΚ1Θ§Βς’ϊ≤ύΝ¥ΝΫΗωΧΦ‘≠Ή”ΚΆτ«ΜυΒΡΈΜ÷ΟΘ§ΖϊΚœΧθΦΰΙ≤”–4÷÷Θ§Ζ÷±π «![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ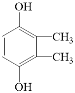 ΚΆ
ΚΆ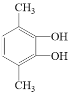 ΓΘ
ΓΘ
Θ®6Θ©ΫαΚœΧβ…ηΩρΆΦΚœ≥…DΒΡΖ¥”Π¬ΖΨΕΘ§ΙΊΦϋ“ΣΚœ≥…““ΥαΦΉθΞCH3COOCH3Θ§άϊ”Ο–≈œΔ÷–ΧαΙ©ΒΡΖ¥”Π±ψΩ…÷Τ±ΗCH3COCH2 COOCH3Θ§“ρΕχ¬ΖœΏ…η÷ΟΈΣ![]()
![]() ΓΘ
ΓΘ


 ΩΎΥψ–ΓΉ¥‘ΣΩΎΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗ
ΩΎΥψ–ΓΉ¥‘ΣΩΎΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗ ΧλΧλΝΖΩΎΥψœΒΝ–¥πΑΗ
ΧλΧλΝΖΩΎΥψœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥ Β―ι–ΓΉιΕ‘FeCl3Ζ÷±π”κNa2SO3ΓΔNaHSO3ΒΡΖ¥”ΠΫχ––ΧΫΨΩΓΘ
Θ®ΦΉΆ§―ßΒΡ Β―ιΘ©
ΉΑ÷Ο | ±ύΚ≈ | ‘ΦΝX | Β―ιœ÷œσ |
| I | Na2SO3»ή“Κ(pHΓ÷9) | ±’ΚœΩΣΙΊΚσΝιΟτΒγΝςΦΤ÷Η’κΖΔ…ζΤΪΉΣ |
II | NaHSO3»ή“Κ(pHΓ÷5) | ±’ΚœΩΣΙΊΚσΝιΟτΒγΝςΦΤ÷Η’κΈ¥ΖΔ…ζΤΪΉΣ |
(1)≈δ÷ΤFeCl3»ή“Κ ±Θ§œ»ΫΪFeCl3»ή”Ύ≈®―ΈΥαΘ§‘ΌœΓ Ά÷Ν÷ΗΕ®≈®Ε»ΓΘΫαΚœΜ·―ß”Ο”οΥΒΟς≈®―ΈΥαΒΡΉς”ΟΘΚΓΘ
(2)ΦΉΆ§―ßΧΫΨΩ Β―ιIΒΡΒγΦΪ≤ζΈο______________ΓΘ
ΔΌ»Γ…ΌΝΩNa2SO3»ή“ΚΒγΦΪΗΫΫϋΒΡΜλΚœ“ΚΘ§Φ”»κ______________Θ§≤ζ…ζΑΉ…Ϊ≥ΝΒμΘ§÷ΛΟς≤ζ…ζΝΥ![]() ΓΘ
ΓΘ
ΔΎΗΟΆ§―ß”÷…ηΦΤ Β―ιΧΫΨΩΝμ“ΜΒγΦΪΒΡ≤ζΈοΘ§Τδ Β―ιΖΫΑΗΈΣ______________ΓΘ
(3) Β―ιI÷–ΗΚΦΪΒΡΒγΦΪΖ¥”Π ΫΈΣ______________ΓΘ
Θ®““Ά§―ßΒΡ Β―ιΘ©
““Ά§―ßΫχ“Μ≤ΫΧΫΨΩFeCl3»ή“Κ”κNaHSO3»ή“ΚΡήΖώΖΔ…ζΖ¥”ΠΘ§…ηΦΤΓΔΆξ≥… Β―ι≤ΔΦ«¬Φ»γœ¬ΘΚ
ΉΑ÷Ο | ±ύΚ≈ | Ζ¥”Π ±Φδ | Β―ιœ÷œσ |
| III | 0~1 min | ≤ζ…ζΚλ…Ϊ≥ΝΒμΘ§”–¥ΧΦΛ–‘ΤχΈΕΤχΧε“ί≥ω |
1~30 min | ≥ΝΒμ―ΗΥΌ»ήΫβ–Έ≥…Κλ…Ϊ»ή“ΚΘ§ΥφΚσ»ή“Κ÷πΫΞ±δΈΣ≥»…ΪΘ§÷°ΚσΦΗΚθΈό…Ϊ | ||
30 minΚσ | ”κΩ’ΤχΫ”¥Ξ≤ΩΖ÷ΒΡ…œ≤ψ»ή“Κ”÷±δΈΣ«≥Κλ…ΪΘ§ΥφΚσ÷πΫΞ±δΈΣ«≥≥»…Ϊ |
(4)““Ά§―ß»œΈΣ¥ΧΦΛ–‘ΤχΈΕΤχΧεΒΡ≤ζ…ζ‘≠“ρ”–ΝΫ÷÷Ω…ΡήΘ§”ΟάκΉ”ΖΫ≥Χ Ϋ±μ ΨΔΎΒΡΩ…Ρή‘≠“ρΓΘ
ΔΌ Fe3+ΘΪ3![]()
![]() Fe(OH)3 ΘΪ3SO2ΘΜΔΎ______________ΓΘ
Fe(OH)3 ΘΪ3SO2ΘΜΔΎ______________ΓΘ
(5)≤ι‘ΡΉ ΝœΘΚ»ή“Κ÷–Fe3+ΓΔ![]() ΓΔOHΘ≠»ΐ÷÷ΈΔΝΘΜα–Έ≥…Κλ…Ϊ≈δΚœΈο≤Δ¥φ‘Ύ»γœ¬ΉΣΜ·ΘΚ
ΓΔOHΘ≠»ΐ÷÷ΈΔΝΘΜα–Έ≥…Κλ…Ϊ≈δΚœΈο≤Δ¥φ‘Ύ»γœ¬ΉΣΜ·ΘΚ
![]()
¥”Ζ¥”ΠΥΌ¬ ΚΆΜ·―ßΤΫΚβΝΫΗωΫ«Ε»Ϋβ Ά1~30 minΒΡ Β―ιœ÷œσΘΚ______________ΓΘ
(6)Ϋβ Ά30 minΚσ…œ≤ψ»ή“Κ”÷±δΈΣ«≥Κλ…ΪΒΡΩ…Ρή‘≠“ρΘΚ______________ΓΘ
Θ® Β―ιΖ¥ΥΦΘ©
(7)Ζ÷±πΕ‘±»IΚΆIIΓΔIIΚΆIIIΘ§FeCl3ΡήΖώ”κNa2SO3ΜρNaHSO3ΖΔ…ζ―θΜ·ΜΙ‘≠Ζ¥”ΠΚΆ”–ΙΊ(–¥≥ωΝΫΧθ)______________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΦν–‘–ΩΟΧΒγ≥ΊΒΡΙΛΉς‘≠άμΘΚZn+2MnO2+2H2O![]() 2MnO(OH)+Zn(OH)2Θ§Τδ÷–ΒΡΒγΫβ÷ »ή“Κ «KOH»ή“ΚΓΘΡ≥ΩΈΧβΉι”ΟΖœΨ…ΧζΩ«ΈόΙ·Φν–‘–ΩΟΧΒγ≥ΊΈΣ‘≠ΝœΘ§÷Τ±Η“Μ÷÷–¬–Ά≤ΡΝœΓΣΓΣMnxZn(1x)Fe2O4Θ§ΤδΙΛ“’Νς≥Χ»γΆΦΥυ ΨΘΚ
2MnO(OH)+Zn(OH)2Θ§Τδ÷–ΒΡΒγΫβ÷ »ή“Κ «KOH»ή“ΚΓΘΡ≥ΩΈΧβΉι”ΟΖœΨ…ΧζΩ«ΈόΙ·Φν–‘–ΩΟΧΒγ≥ΊΈΣ‘≠ΝœΘ§÷Τ±Η“Μ÷÷–¬–Ά≤ΡΝœΓΣΓΣMnxZn(1x)Fe2O4Θ§ΤδΙΛ“’Νς≥Χ»γΆΦΥυ ΨΘΚ

(1)“―÷ΣMnxZn(1x)Fe2O4÷–ΟΧ‘ΣΥΊΒΡΜ·ΚœΦέ”κ Β―ι “”ΟΕΰ―θΜ·ΟΧ÷Τ»Γ¬»Τχ ±ΜΙ‘≠≤ζΈο÷–ΒΡΟΧœύΆ§Θ§‘ρΧζ‘ΣΥΊΒΡΜ·ΚœΦέΈΣ___________ΓΘ
(2)ΓΑ»ή‘ϋΓ±ΙΛ–ρ÷–œΓΝρΥα”κΧζΖ¥”Π…ζ≥…ΒΡΝρΥα―«ΧζΩ…ΫΪ+3ΦέΟΧΒΡΜ·ΚœΈο»Ϊ≤ΩΜΙ‘≠≥…Mn2+Θ§–¥≥ωΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ_________________________________ΓΘ
(3)ΓΑΒςΧζΓ±ΙΛ–ρΒΡΡΩΒΡ «Βς’ϊ¬Υ“Κ÷–ΧζάκΉ”ΒΡΉή≈®Ε»Θ§ ΙΤδ÷–Ϋπ τ‘ΣΥΊΒΡΈο÷ ΒΡΝΩ÷°±»”κ≤ζΤΖΒΡΜ·―ß ΫMnxZn(1x)Fe2O4œύΖϊΚœΓΘ
ΔΌ–¥≥ωΓΑΒςΧζΓ±ΙΛ–ρ÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ______________________ΓΔ_______ΓΘ
ΔΎ»τ≤βΒΟ¬Υ“ΚΒΡ≥…Ζ÷ΈΣc(Mn2+)+c(Zn2+)=a molΓΛL1Θ§c(Fe2+)+c(Fe3+)=b molΓΛL1Θ§¬Υ“ΚΧεΜΐΈΣ1 m3Θ§ΓΑΒςΧζΓ±ΙΛ–ρ÷–Θ§–ηΦ”»κΒΡΧζΖέ÷ ΝΩΈΣ___________kg(Κω¬‘»ή“ΚΧεΜΐ±δΜ·Θ§”ΟΚ§aΓΔbΒΡ¥ζ ΐ Ϋ±μ Ψ)ΓΘ
(4)‘ΎΓΑ―θΜ·Γ±ΙΛ–ρ÷–Θ§Φ”»κΥΪ―θΥ°ΒΡΡΩΒΡ «Α― Fe 2+ ―θΜ·ΈΣ Fe 3+ΘΜ…ζ≤ζΙΐ≥Χ÷–ΖΔœ÷ ΒΦ œϊΚΡΥΪ―θΥ°ΒΡΝΩ¥σ”Ύάμ¬έ÷ΒΘ§ΤδΩ…Ρή‘≠“ρ≥ΐΈ¬Ε»ΆβΘ§÷ς“Σ «______________________ΓΘ
(5)”ΟΑ±Υ°ΓΑΒςpHΓ±ΚσΘ§Ψ≠ΓΑΫαΨßΓ±ΓΔΓΑΙΐ¬ΥΓ±Ω…ΒΟΒΫ≤ζΤΖΚΆ¬Υ“ΚCΘ§¥”¬Υ“ΚC÷–ΜΙΩ…Ζ÷άκ≥ω“Μ÷÷ΒΣΖ Θ§ΗΟΒΣΖ ΒΡ»ή“Κ÷–άκΉ”≈®Ε»”…¥σΒΫ–ΓΒΡ≈≈–ρΈΣ_______________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ≥ΘΈ¬œ¬Θ§œρΡ≥≈®Ε»ΒΡH2C2O4»ή“Κ÷–÷πΒΈΦ”»κ“―÷Σ≈®Ε»ΒΡNaOH»ή“ΚΘ§»τpC±μ Ψ»ή“Κ÷–»ή÷ ΈΔΝΘΒΡΈο÷ ΒΡΝΩ≈®Ε»ΒΡΗΚΕ‘ ΐΘ§‘ρΥυΒΟ»ή“Κ÷–pC(H2C2O4)Θ§pC(HC2O4Θ≠)ΓΔpC(C2O42Θ≠)”κ»ή“ΚpHΒΡ±δΜ·ΙΊœΒ»γΆΦΥυ ΨΓΘ“―÷ΣΘΚH2C2O4![]() HC2O4Θ≠+H+ Ka1ΘΜHC2O4Θ≠
HC2O4Θ≠+H+ Ka1ΘΜHC2O4Θ≠![]() C2O42Θ≠+H+ Ka2ΓΘ‘ρœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
C2O42Θ≠+H+ Ka2ΓΘ‘ρœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «

A. Β±pH=3 ±Θ§»ή“Κ÷–c(HC2O4Θ≠)<c(C2O42Θ≠)=c(H2C2O4)
B. pH”…3‘ω¥σΒΫ5.3ΒΡΙΐ≥Χ÷–Θ§Υ°ΒΡΒγάκ≥ΧΕ»÷πΫΞΦθ–Γ
C. ≥ΘΈ¬œ¬Θ§Ka2=10Θ≠5.3
D. ≥Θœ¬ΥφΉ≈pHΒΡ‘ω¥σΘΚc2(HC2O4Θ≠)/[c(H2C2O4)c(C2O42Θ≠)] ΒΡ÷Βœ»‘ω¥σΚσΦθ–Γ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ»γΆΦΦΉ «άϊ”Ο“Μ÷÷ΈΔ…ζΈοΫΪΖœΥ°÷–ΒΡΡρΥΊ[CO(NH2)2]ΒΡΜ·―ßΡή÷±Ϋ”ΉΣΜ·ΈΣΒγΡήΘ§≤Δ…ζ≥…ΜΖΨ≥”―ΚΟΈο÷ ΒΡΉΑ÷ΟΘ§Ά§ ±άϊ”Ο¥ΥΉΑ÷ΟΒΡΒγΡή‘ΎΧζ…œΕΤΆ≠ΓΘœ¬Ν–ΥΒΖ®÷–’ΐ»ΖΒΡ «
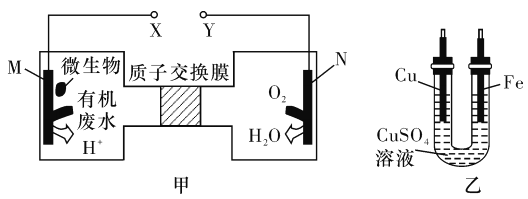
A. Ά≠ΒγΦΪ”Π”κXœύΝ§Ϋ”
B. H+Ψ≠Ιΐ÷ Ή”ΫΜΜΜΡΛ”…”“œρΉσ“ΤΕ·
C. Β±NΒγΦΪœϊΚΡ0. 25 molΤχΧε ±Θ§‘ρΧζΒγΦΪ‘ω÷Ί16 g
D. MΒγΦΪΖ¥”Π ΫΘΚCO(NH2)2+H2O-6e- =CO2Γϋ+N2Γϋ+6H+
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΧλ»ΜΥ°¥σΕύΚ§Ca2+ΓΔMg2+ΓΔHCO3-Β»άκΉ”Θ§Φ”»»Μα≤ζ…ζΥ°ΙΗΘ§Υ°ΙΗ÷–“ΜΕ®Κ§”–CaCO3ΚΆMg(OH)2Θ§Ω…ΡήΚ§”–MgCO3ΓΘ
(1)Χλ»ΜΥ°÷–ΒΡHCO3-ά¥Ή‘”ΎΩ’Τχ÷–ΒΡCO2ΓΘ”ΟœύΙΊΖΫ≥Χ Ϋ±μ ΨCO2»ή”ΎΥ°–Έ≥…HCO3-ΒΡΙΐ≥ΧΓΘ______________________________________________________________
(2)Χλ»ΜΥ°÷σΖ– ±Θ§Τδ÷–ΈΔ»ήΒΡMgCO3ΉΣΜΜ≥…Ρ―»ήΒΡMg(OH)2Θ§–¥≥ωΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΓΘ____________________________________________
ΈΣ»ΖΕ®Ρ≥Υ°ΙΗ―υΤΖΒΡ≥…Ζ÷Θ§άϊ”ΟCaCO3ΓΔMgCO3ΓΔMg(OH)2ΗΏΈ¬Ζ÷ΫβΒΡ–‘÷ Θ§ΨΪ»Ζ≥ΤΝΩ5.000gΥ°ΙΗ―υΤΖΘ§”Ο»γœ¬ΆΦΉΑ÷ΟΫχ–– Β―ιΓΘ
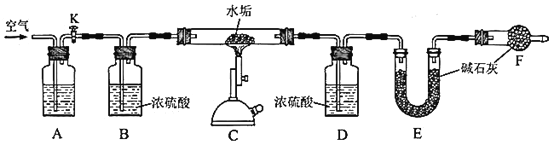
(3)A÷– ΔΖ≈ΒΡ ‘ΦΝ «__________ΓΘΉΑ÷ΟFΒΡΉς”Ο «_________________________ΓΘΖ¥”ΠΫα χΚσΘ§–η“Σ‘ΌΆ®»κ“ΜΕΈ ±ΦδΒΡΩ’ΤχΘ§ΡΩΒΡ «_______________________ΓΘ
(4)άϊ”Ο…œ ωΉΑ÷Ο≤βΕ®Υ°ΙΗ÷–Mg(OH)2ΒΡΚ§ΝΩ ±Θ§–η“Σ≤βΝΩΒΡ ΐΨί”–__________ΓΘ
(5) Β―ι≤βΒΟΉΑ÷ΟE‘ω÷Ί2.200gΘ§«κΈ Υ°ΙΗ―υΤΖ÷– «ΖώΚ§”–MgCO3?≈–Εœ“άΨί «_______________________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΆ®ΙΐΚΘΥ°Νά…ΙΩ…ΒΟ¥÷―Έ,¥÷―Έ÷–≥ΐΚ§”–NaClΆβ,ΜΙΚ§”–![]() “‘ΦΑΡύ…≥Β»‘”÷ ΓΘ÷Τ±ΗΨΪ―ΈΒΡΗς≤Ϋ≤ΌΉςΝς≥Χ»γœ¬:
“‘ΦΑΡύ…≥Β»‘”÷ ΓΘ÷Τ±ΗΨΪ―ΈΒΡΗς≤Ϋ≤ΌΉςΝς≥Χ»γœ¬:

(1)‘ΎΒΎΔΏ≤Ϋ’τΖΔΙΐ≥Χ÷–“Σ”Ο≤ΘΝßΑτΫΝΑη,ΡΩΒΡ «__________ΓΘ
(2)ΒΎΔΎΓΔΔή≤Ϋ≤ΌΉςΒΡΡΩΒΡΖ÷±π «≥ΐ»Ξ¥÷―Έ÷–ΒΡ![]() ΚΆ
ΚΆ![]() ,–η“ΣΦ”»κΒΡ ‘ΦΝ“ά¥Έ «________(ΧνΜ·―ß Ϋ)ΓΘ
,–η“ΣΦ”»κΒΡ ‘ΦΝ“ά¥Έ «________(ΧνΜ·―ß Ϋ)ΓΘ
(3)ΒΎΔό≤Ϋ≤ΌΉς÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ________ΓΘ
(4)‘ΎΒΎΔέ≤Ϋ≤ΌΉς÷–,―Γ‘ώΒΡ≥ΐ‘” ‘ΦΝ≤ΜΡή «KOH»ή“Κ,άμ”… «_____________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΚΘΥ° «Ψό¥σΒΡΉ ‘¥±ΠΩβΘ§ΚΘΥ°Β≠Μ·ΦΑΤδΉέΚœάϊ”ΟΨΏ”–÷Ί“Σ“β“εΓΘ

«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©≤Ϋ÷ηI÷–Θ§¥÷―Έ÷–Κ§”–Ca2+ΓΔMg2+ΓΔSO42-Β»‘”÷ άκΉ”Θ§¥÷―ΈΨΪ÷ΤΙΐ≥Χ÷–“Σ Ι”ΟNa2CO3»ή“ΚΘ§«κ–¥≥ωΦ”»κNa2CO3»ή“ΚΚσœύΙΊΜ·―ßΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ_________________________ΓΘ
Θ®2Θ©ΚΘΥ°ΧαδεΘ§÷ΤΒΟ1molBr2÷Ν…Ό–η“ΣœϊΚΡ_________molCl2ΓΘ≤Ϋ÷ηΔσ»τ”ΟNa2SO3Υ°»ή“ΚΈϋ ’Br2Θ§”–ΙΊΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ_________ΓΘ
Θ®3Θ©”ΟΥΡ¬»Μ·ΧΦΩ…“‘ΫΪ…ζ≥…ΒΡδεΧα»Γ≥ωά¥Θ§άϊ”ΟΝΥδεΒΡ________–‘÷ Θ§ΈΣΝΥ≥ΐ»Ξ≤ζΈο÷–≤–ΝτΒΡ…ΌΝΩCl2Θ§Ω…œρΤδ÷–Φ”»κ_________»ή“ΚΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ“―÷ΣΘΚp[c(HX)/c(X-)]=-lg[c(HX)/c(X-)]ΓΘ “Έ¬œ¬Θ§œρ0. 10 mol/LHX»ή“Κ÷–ΒΈΦ”0.10 mol/L NaOH»ή“ΚΘ§»ή“ΚpHΥφp[c(HX)/c(X-)]±δΜ·ΙΊœΒ»γΆΦΓΘœ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «
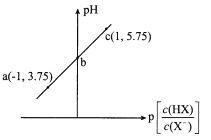
A. »ή“Κ÷–Υ°ΒΡΒγάκ≥ΧΕ»ΘΚa<b<cB. ΆΦ÷–bΒψΉχ±ξΈΣ(0Θ§4.75)
C. cΒψ»ή“Κ÷–ΘΚc(Na+) =l0c(HX)D. “Έ¬œ¬HXΒΡΒγάκ≥Θ ΐΈΣ10-4. 75
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com