
����Ŀ�����ǻ��ʹ�ҵ�ͻ����л���������Ҫԭ�ϡ�
��1���ϳɰ���Ӧ�������й����ʵĻ�ѧ�������������±���ʾ��
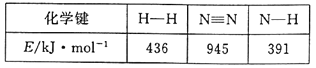
��д���úϳɰ���Ӧ���Ȼ�ѧ����ʽ___��
��2��һ���¶��£��ϳɰ���Ӧ��a��b���������·ֱ�ﵽƽ�⣬H2��Ũ����ʱ��ı仯��ͼ��ʾ��
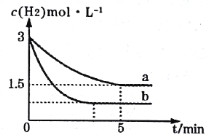
��a�����£�0��5min�ڵ�ƽ����Ӧ����v(N2)=___mol��L-1��min-1��
�����a���ԣ�b���ܸı��������___��
��3��ij��ѧ��ȤС����һ�����ܱ������г���4molN2��12molH2ģ��ϳɰ���Ӧ��ƽ�������а����������������ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ��
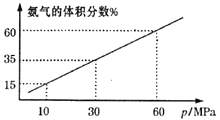
����ϵ��60MPa�´ﵽƽ�⡣H2��ƽ���ѹΪ___MPa��(��ѹ=��ѹ�����ʵ�������)����ʽ������ʱ��ƽ�ⳣ��Kp=___��(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬�������2λ��Ч����)
��4������ͼʾ���ܱ�ʾ�ϳɰ���Ӧ�ں��¡������ܱ���������t1ʱ���Ѿ��ﵽƽ��״̬����___��

���𰸡�N2(g)+3H2(g)![]() 2NH3(g)��H=-93kJ��mol-1 0.1mol��L-1��min-1 ����N2Ũ�� 18 0.037 b
2NH3(g)��H=-93kJ��mol-1 0.1mol��L-1��min-1 ����N2Ũ�� 18 0.037 b
��������
(1)�ɼ��������ȣ�945 kJ +3��436 kJ=2253kJ���½��ϳɷ��ȣ�6��391 kJ=2346kJ�����ȶ࣬��ѧ��Ӧ����Ϊ���ȣ��ų�����93kJ���Ȼ�ѧ����ʽΪN2(g)+3H2(g)![]() 2NH3(g)��H=-93kJ��mol-1��
2NH3(g)��H=-93kJ��mol-1��
��2��a�����£�0��5min�ڵ�ƽ����Ӧ����v(H2)=![]() ������ϵ���ȿ�֪v(N2)=0.1mol��L-1��min-1��
������ϵ���ȿ�֪v(N2)=0.1mol��L-1��min-1��
a��b������ʼŨ����ͬ��b����ƽ���ʱ�����̣�˵����Ӧ��������ƽ��ʱ������Ũ�ȼ�С��˵��ƽ�������ƶ������Ըı������������c��N2����
��3��������ϵ��60MPa�´ﵽƽ�⣬��ͬ�¶��£����������������������ʵ����ķ�������

ƽ��ʱ�����������=![]()
���x=3
H2��ƽ���ѹ=![]()
��������ƽ�����ݼ���Kp![]()
��4��a.�������ʼ�����������غ㣬�����������䲻��ƽ��ı�־��
b.N2�����������˵���Ѿ�ƽ�⣻
c.�������ʼ�����������غ㣬������������ܶȲ��䲻��ƽ��ı�־��
d.��ѧƽ�ⳣ��ֻ���¶��йأ�����ƽ��ı�־��
��ѡb��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��һ�ֺ�̼���⡢������Ԫ�ص��л��������֪��A��̼����������Ϊ44.1%�������������Ϊ8.82%��Aֻ��һ�ֹ����ţ���ÿ��̼ԭ�������ֻ��һ�������ţ�A�������ᷢ��������Ӧ������������������̼ԭ���Ϸ�����ȥ��Ӧ��ͨ��������
��1��A�ķ���ʽ��_____________��д��������̣�����ṹ��ʽ��____________________��
��2��д��A�����ᷴӦ�Ļ�ѧ����ʽ��________________________________________��
��3��27.2gA�ڿ����г��ȼ��ʱ������Ҫ����������£�����L�����ɵ�CO2��170ml 10mol/L��NaOH��Һ���գ���Ӧ����Һ�����ʵijɷ���____�������ʵ�������______��
��4��д��������������3��������A��ͬ���칹��Ľṹ��ʽ������ֱ�����������A������ͬ�Ĺ����ţ���ÿ��̼ԭ�������ֻ��һ�������š�д����Щͬ���칹��Ľṹ��ʽ_______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
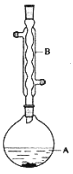
����Ŀ������������������۷���Ϣ���ʵijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧװ��ʾ��ͼ���й��������£�
![]() +H2O
+H2O
���ԭ������ | �ܶ�/��g��cm-3�� | �е�/�� | ˮ���ܽ��� | |
���촼 | 88 | 0.8123 | 131 | �� |
���� | 60 | 1.0492 | 118 | �� |
���������� | 130 | 0.8670 | 142 | ���� |
ʵ�鲽�裺
��A�м���4.4g�����촼��6.0g�����ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50���ӣ���ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮ����þ���壬����Ƭ�̣����˳�ȥ����þ���壬�������������ռ�140��143����֣�������������3.9g���ش��������⣺
��1��װ��B�������ǣ�___��
��2���÷�Ӧ��Ũ���������___������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ___��
��3���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ����___��
��4����ϴ�Ӳ����У���һ��ˮϴ����ҪĿ���ǣ�___���ڶ���ˮϴ����ҪĿ���ǣ�___��
��5����ʵ���м�����������Ŀ���ǣ�___��
��6������������У�����ѡ��װ����ȷ���ǣ�___(����)
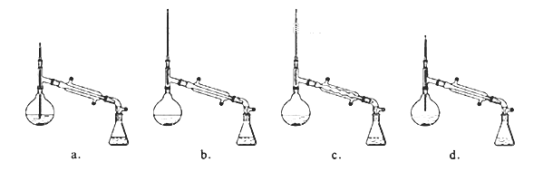
��7����ʵ��IJ�����___��
A.30�G B.40�G C.50�G D.60�G
��8���ڽ����������ʱ������130�濪ʼ�ռ���֣�����ƫ___(����ߵ�)ԭ����___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶�ʱ��Ag2SO4��ˮ��Һ�еij����ܽ�ƽ��������ͼ��ʾ������˵����ȷ���ǣ� ��
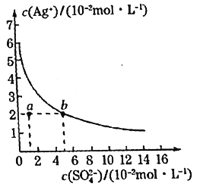
A.���д���SO42-����Һ�п϶�������Ag+
B.���¶��£�Ag2SO4���ܶȻ�����(Ksp)����������3
C.����ͨ���ı��¶Ȼ��������������ʹa���ƶ���b��
D.���¶��£�0.02mol��L-1��AgNO3��Һ��0.2mol��L-1��Na2SO4��Һ�������ϣ��������ɳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2019��ŵ�������������ڿ�������ӵ�ط�������Խ������λ��ѧ�ҡ�����ӵ�صĹ㷺Ӧ��Ҫ����﮵�ط����Խ�Լ��Դ����������������Ӷ��ε����������Ĥ��Ҫ����LiCoO2��Al�ȣ������÷��ϵ�һ�ֹ�����ͼ��ʾ��

��1��Li��ԭ�ӽṹʾ��ͼΪ_____________��LiCoO2��Co�Ļ��ϼ�Ϊ_______��
��2���������ʱAl�ܽ�����ӷ���ʽΪ__________________________________��
��3�������ܡ�ʱ����H2O2��Ŀ����______________________________________��
��4�������ܡ������ӷ���ʽΪ___________________________________________��
��5������100 mL 1.0 mol/L (NH4)2C2O4��Һ����Ҫ�IJ������������������ձ��⣬����_____��
��6��ȡCoC2O4����4.41g�ڿ����м�����300 �棬�õ����ܵ�������2.41g����÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����20 mL 0.1 mol��L��1һԪ����HA��Һ�еμ�0. 1 mol�� L��1 NaOH��Һ����Һ��1g[c(A-)/c(HA)]��pH��ϵ��ͼ��ʾ������˵����ȷ����

A. A���Ӧ��Һ�У�c(Na+)��c(A-)��c(H+)��c(OH-)
B. 25��ʱ��HA��ĵ��볣��Ϊ1. 0�� 10��5.3
C. B���Ӧ��NaOH��Һ���Ϊ10 mL
D. ��C����Һ����(�����ǻӷ�)����c(A-)/[c(HA)c(OH-)]һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼװ�ã����������º�һ��������ѹ�����²ⶨþ�����ԭ��������Y����һ���þ��һ���ϡ���ᣬ��бY�ܣ���ϡ����ȫ��������һ�࣬��Ӧ��ʼ��
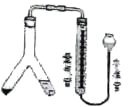
(1)��Y���������Լ�ʱ��Ҫȷ��________������
(2)��Ӧǰ��Ҫ���������ܺ�ˮ��Һ����ƽ���Ӷ���֤_____��ȡ�������ʱ���������е�Һ�����ˮ�ܵ�Һ�棬ʵ����H2�������______��(����ƫ������ƫС������������)
(3)��þ������Ϊm g��c mol/L������ҺV mL����Ӧ���ռ������������Ϊa mL(������Ϊ��״����)����þ�����ԭ������Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���⻯�ⳣ�����Ʊ���Ļ�����������л���Ӧ�Ļ�ԭ�����⻯�ⲻ�ȶ����ֽ⣬�⻯�⼫������ˮ����ˮ��Һ��Ϊ����ᣬ�������ǿ�ᣬ�н�ǿ�Ļ�ԭ�ԡ�
(1)��������ڿ����г��ڷ��ã���Һ���ɻ�ɫ����ԭ����___���û�ѧ����ʽ����ʾ����
(2)ʵ�����г��ø���ĺ��͵���Ӵ���������ˮ�ȣ��������ɵ⻯��������ᣨH3PO3�����÷�Ӧ�Ļ�ѧ����ʽΪ___��
(3)�����͵�������ֱ�ӷ�Ӧ���ɵ⻯�⣬H2(g)��I2(g)![]() 2HI(g) ��H��0��T��ʱ����1L�����ܱ������г���0.2molH2��0.2molI2(g)��5minʱ��Ӧ�ﵽƽ�⣬H2��I2(g)��HI�����ʵ���Ũ��(c)��ʱ��(t)�仯��������ͼl��ʾ��
2HI(g) ��H��0��T��ʱ����1L�����ܱ������г���0.2molH2��0.2molI2(g)��5minʱ��Ӧ�ﵽƽ�⣬H2��I2(g)��HI�����ʵ���Ũ��(c)��ʱ��(t)�仯��������ͼl��ʾ��
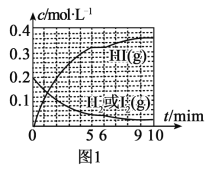
��0��5min�ڣ���HI��ʾ�ĸ÷�Ӧ����v(HI)=___��
��T��ʱ���÷�Ӧ��ƽ�ⳣ��K=___��
��6minʱ���ı���������Ϊ___��
��10minʱ�����������������䣬���������г���0.1molH2��0.1molI2(g)��0.2molHI(g)��12minʱ�ﵽ��ƽ�⡣��ͼ2�л���10��12min��H2��HI��Ũ�ȱ仯����___�������ϱ���H2��HI����0��5min��0��2minʱ��Σ�H2��ת���ʷֱ�����1����2��ʾ������l___��2�����������������=������
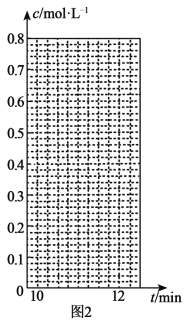
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���������Ҫ�ɷ�ΪAl2O3��xH2O��������Fe2O3��SiO2���ʡ���ȡ17.5g��������Ʒ������200mL1.65mol/LϡH2SO4��ǡ����ȫ��Ӧ�����˵õ�����0.3g��Ȼ������Һ�м�������NaOH��Һ���õ�����2.14g������ʾ��SiO2�Ӳ���ϡ���ᷴӦ��
��1��д�������漰��������������Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ___��
��2������Ʒ�У�Fe2O3�����ʵ���___��
��3����������Ʒ��Al2O3������___��
��4���Լ�����Ʒ��Al2O3��xH2O��xֵ___����Ҫ�������̣�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com