
����Ŀ���⻯�ⳣ�����Ʊ���Ļ�����������л���Ӧ�Ļ�ԭ�����⻯�ⲻ�ȶ����ֽ⣬�⻯�⼫������ˮ����ˮ��Һ��Ϊ����ᣬ�������ǿ�ᣬ�н�ǿ�Ļ�ԭ�ԡ�
(1)��������ڿ����г��ڷ��ã���Һ���ɻ�ɫ����ԭ����___���û�ѧ����ʽ����ʾ����
(2)ʵ�����г��ø���ĺ��͵���Ӵ���������ˮ�ȣ��������ɵ⻯��������ᣨH3PO3�����÷�Ӧ�Ļ�ѧ����ʽΪ___��
(3)�����͵�������ֱ�ӷ�Ӧ���ɵ⻯�⣬H2(g)��I2(g)![]() 2HI(g) ��H��0��T��ʱ����1L�����ܱ������г���0.2molH2��0.2molI2(g)��5minʱ��Ӧ�ﵽƽ�⣬H2��I2(g)��HI�����ʵ���Ũ��(c)��ʱ��(t)�仯��������ͼl��ʾ��
2HI(g) ��H��0��T��ʱ����1L�����ܱ������г���0.2molH2��0.2molI2(g)��5minʱ��Ӧ�ﵽƽ�⣬H2��I2(g)��HI�����ʵ���Ũ��(c)��ʱ��(t)�仯��������ͼl��ʾ��
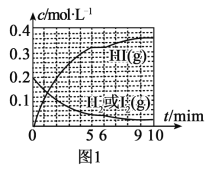
��0��5min�ڣ���HI��ʾ�ĸ÷�Ӧ����v(HI)=___��
��T��ʱ���÷�Ӧ��ƽ�ⳣ��K=___��
��6minʱ���ı���������Ϊ___��
��10minʱ�����������������䣬���������г���0.1molH2��0.1molI2(g)��0.2molHI(g)��12minʱ�ﵽ��ƽ�⡣��ͼ2�л���10��12min��H2��HI��Ũ�ȱ仯����___�������ϱ���H2��HI����0��5min��0��2minʱ��Σ�H2��ת���ʷֱ�����1����2��ʾ������l___��2�����������������=������
���𰸡�4HI��O2=2H2O��2I2 2P��3I2��6H2O=2H3PO3��6HI 0.064mol��L-1��min-1 64 ���� 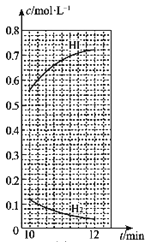 ��
��
��������
(1)�������н�ǿ�Ļ�ԭ�ԣ�¶���ڿ����лᱻ�����е���������Ϊ�ⵥ�ʣ��Ӷ�ʹ��Һ��ƣ���Ӧ�Ļ�ѧ����ʽΪ��4HI��O2=2H2O��2I2����Ϊ��4HI��O2=2H2O��2I2��
(2)���������������ԭ��Ӧ����ʽ����ƽԭ�÷�Ӧ�Ļ�ѧ����ʽΪ��2P��3I2��6H2O=2H3PO3��6HI����Ϊ��2P��3I2��6H2O=2H3PO3��6HI��
(3)����ͼ���֪��0��5min�ڣ���c(HI)=0.32mol��L-1��v(HI)=![]() =0.064mol��L-1��min-1����Ϊ��0.064mol��L-1��min-1��
=0.064mol��L-1��min-1������0.064mol��L-1��min-1��
��T��ʱ���÷�Ӧ��5minʱ�ﵽƽ�⣬��ͼ���֪��ƽ��ʱ��c(HI)=0.32mol��L-1��c(H2)=c(I2)=0.04mol��L-1����T��ʱ�÷�Ӧ��ƽ�ⳣ��K=![]() =
=![]() =64������64��
=64������64��
��6minʱ����Ӧ�����Ũ��˲�䲻�䣬�����ŷ�Ӧ�Ľ��У���Ӧ��Ũ�ȼ��٣�������Ũ�����ӣ�˵��ƽ�������ƶ������ڸ�����ӦΪ���ȷ�Ӧ����6minʱ�ı�������ǽ����¶ȡ���Ϊ�����£�
��10minʱ���Ѵﵽ�µ�ƽ�⣬��ʱc(H2)=c(I2)=0.02mol��L-1��c(HI)=0.36mol��L-1����K=![]() =
=![]() =324�����������������䣬���������г���0.1molH2��0.1molI2(g)��0.2molHI����ʱ��c(H2)=c(I2)=0.12mol��L-1��c(HI)=0.56mol��L-1��Qc=
=324�����������������䣬���������г���0.1molH2��0.1molI2(g)��0.2molHI����ʱ��c(H2)=c(I2)=0.12mol��L-1��c(HI)=0.56mol��L-1��Qc=![]() =
=![]() =
=![]() ��K=324��ƽ�������ƶ���c(H2)��c(I2)��С��c(HI)����12minʱ����Ӧ�ﵽ��ƽ�⣬��10min��12min��������c(H2)=��c(I2)=xmol��L-1����12minʱ��c(H2)=c(I2)=(0.12��x)mol��L-1��c(HI)=(0.56��2x)mol��L-1����K=
��K=324��ƽ�������ƶ���c(H2)��c(I2)��С��c(HI)����12minʱ����Ӧ�ﵽ��ƽ�⣬��10min��12min��������c(H2)=��c(I2)=xmol��L-1����12minʱ��c(H2)=c(I2)=(0.12��x)mol��L-1��c(HI)=(0.56��2x)mol��L-1����K=![]() =
=![]() =324����ã�x=0.08����12minʱ��c(H2)=c(I2)=(0.12��0.08)mol��L-1=0.04mol��L-1��c(HI)=(0.56��0.16)mol��L-1=0.72mol��L-1����10��12min��H2��HI��Ũ�ȱ仯������ͼ
=324����ã�x=0.08����12minʱ��c(H2)=c(I2)=(0.12��0.08)mol��L-1=0.04mol��L-1��c(HI)=(0.56��0.16)mol��L-1=0.72mol��L-1����10��12min��H2��HI��Ũ�ȱ仯������ͼ ��ʾ��
��ʾ��
��ͼʾ��֪��0��5minʱ��H2��ת������1=![]() ��100%=80%��0��12minʱ���������n(H2)=0.3mol��12minʱ��ʣ��n(H2)=0.04mol��L-1��1L=0.04mol����0��12minʱ��H2��ת������2=
��100%=80%��0��12minʱ���������n(H2)=0.3mol��12minʱ��ʣ��n(H2)=0.04mol��L-1��1L=0.04mol����0��12minʱ��H2��ת������2=![]() ��100%��86.7%������1����2����Ϊ��
��100%��86.7%������1����2������ ������
������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
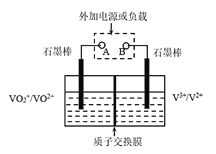
����Ŀ��ȫ��Һ�����ܵ�������ò�ͬ��̬���ӶԵ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ��(��ͼ)��
��֪��
����Һ��������������ΪSO42-��
����Һ����ɫ��V3+��ɫ��V2+��ɫ��VO2+��ɫ��VO2+��ɫ��
�۷ŵ�����У��Ҳ���Һ����ɫ����ɫ�����ɫ��
����˵������ȷ����
A.�ŵ�ʱB��Ϊ����
B.�ŵ�ʱ��ת�Ƶĵ�����Ϊ3.01��1023�����������H+����0.5 mol
C.����������۵ĵ缫��ӦʽΪ��VO2++H2O-e- =VO2++2H+
D.��������H+ͨ�����ӽ���Ĥ���Ҳ��ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǻ��ʹ�ҵ�ͻ����л���������Ҫԭ�ϡ�
��1���ϳɰ���Ӧ�������й����ʵĻ�ѧ�������������±���ʾ��
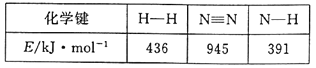
��д���úϳɰ���Ӧ���Ȼ�ѧ����ʽ___��
��2��һ���¶��£��ϳɰ���Ӧ��a��b���������·ֱ�ﵽƽ�⣬H2��Ũ����ʱ��ı仯��ͼ��ʾ��
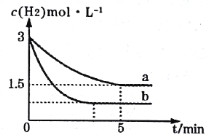
��a�����£�0��5min�ڵ�ƽ����Ӧ����v(N2)=___mol��L-1��min-1��
�����a���ԣ�b���ܸı��������___��
��3��ij��ѧ��ȤС����һ�����ܱ������г���4molN2��12molH2ģ��ϳɰ���Ӧ��ƽ�������а����������������ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ��
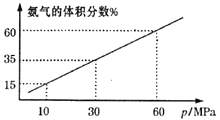
����ϵ��60MPa�´ﵽƽ�⡣H2��ƽ���ѹΪ___MPa��(��ѹ=��ѹ�����ʵ�������)����ʽ������ʱ��ƽ�ⳣ��Kp=___��(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬�������2λ��Ч����)
��4������ͼʾ���ܱ�ʾ�ϳɰ���Ӧ�ں��¡������ܱ���������t1ʱ���Ѿ��ﵽƽ��״̬����___��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣����ڱ���Ԫ�آ١��࣬��ջش�
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ����A | 0 |
�� | �� | �� | �� | �� | ||||
�� | �� | �� | �� | �� |
(1)�ؿ��к�������Ԫ����______���ǽ�������ǿ��Ԫ����______��
(2)д���ٵ������̬�⻯��ĵ���ʽ______��
(3)�ڵ�����������Ԫ���У�������������ǿ����_____�����γɵĶ�Ԫǿ����________��
(4)д���ڵ���̬�⻯����ڵ�����������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����100mL1.5mol��L-1��Na2CO3��Һ��ͨ���״����1.12LCO2���壬��ַ�Ӧ����������Һ��������䣬��������Һ�У������й����ӵĹ�ϵ��ȷ���ǣ� ��
A.c(Na+)��c(CO32-)��c(HCO3-)��c(OH-)��c(H+)
B.c(Na+)=c(HCO3-)��2c(CO32-)
C.3c(H2CO3)��c(HCO3-)��2c(H+)=c(CO32-)��2c(OH-)
D.c(HCO3-)��c(CO32-)=2mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ߴ���������Ϊ�ϳ���������Ԫ�������ϵ�ԭ�ϣ���ҵ�Ͽ�����Ȼ�������̷������̿���Fe��Al��Mg��Zn��Ni��Si��Ԫ�أ��Ʊ�����������ͼ��ʾ���ش��������⣺

��ؽ�������[c0(Mn+)=0.1 mol��L1]�γ��������������pH��Χ���£�
�������� | Mn2+ | Fe2+ | Fe3+ | Al3+ | Mg2+ | Zn2+ | Ni2+ |
��ʼ������pH | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 | 6.2 | 6.9 |
������ȫ��pH | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 | 8.2 | 8.9 |
��1��������1������S��__________________________��д�����ܽ����ж������������̷�Ӧ�Ļ�ѧ����ʽ_______________________________________��
��2����������������������MnO2�������ǽ�________________________���������������Լ�___________��
��3������pH��������������Һ��pH��ΧӦ����Ϊ_______��6֮�䣬���������ӷ�Ӧ����ʽ��__________��
��4��������1����Ŀ���dz�ȥZn2+��Ni2+��������3������Ҫ�ɷ���______________��
��5�������г�����������MnO2��������H2O2��������д�������ӷ�Ӧ_______________��
��6��д�������̡������ӷ���ʽ_______________________________________��
��7����״��������Ԫ���Ͽ���Ϊ����ӵ���������ϣ��仯ѧʽΪLiNixCoyMnzO2������Ni��Co��Mn�Ļ��ϼ۷ֱ�Ϊ+2��+3��+4����x=y=![]() ʱ��z=___________��
ʱ��z=___________��
��8��д��Fe2+��HNO3�����ӷ�Ӧ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������µ��ܱ������У�4NH3(g)��5O2(g)![]() 4NO(g)��6H2O(g) ��H����905.9 kJ/mol������������ȷ����
4NO(g)��6H2O(g) ��H����905.9 kJ/mol������������ȷ����
A.�������г���4 mol NH3��5 mol O2���з�Ӧ���ﵽƽ��ʱ�ų�������Ϊ905.9 kJ��
B.ƽ��ʱv��(O2)��v��(NO)��
C.ƽ���ѹǿ���������ƽ��Ħ����������
D.ƽ��������¶ȣ����������NO�������͡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��Ӧ��2A��B![]() 2C������A��B��C��Ϊ���壬��ͼ�е������Ǹ÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�����ߣ�x���ʾ�¶ȣ�y���ʾB��ת���ʣ�ͼ���� a��b��c���㣬��ͼ��ʾ��������������ȷ����
2C������A��B��C��Ϊ���壬��ͼ�е������Ǹ÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�����ߣ�x���ʾ�¶ȣ�y���ʾB��ת���ʣ�ͼ���� a��b��c���㣬��ͼ��ʾ��������������ȷ����
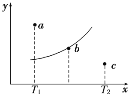
A.�÷�Ӧ�Ƿ��ȷ�Ӧ
B.b��ʱ��������ƽ��Ħ���������ٱ仯
C.T1�¶�������a��ﵽƽ�⣬���Բ�ȡ����ѹǿ�ķ���
D.c�㣺v(��)<v(��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���߷��Ӳ��� PET ������֬�� PMMA �ĺϳ�·�����£�
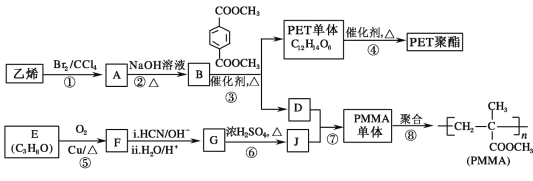
��֪����.RCOOR����R��18OH RCO18OR����R��OH(R��R����R����������)
RCO18OR����R��OH(R��R����R����������)
II.  (R��R����������)
(R��R����������)
(1)�ٵķ�Ӧ������_____________��
(2)�ڵĻ�ѧ����ʽΪ____________��
(3)PMMA ���������������_____��_____��
(4)F �ĺ˴Ź���������ʾֻ��һ��壬�ݵĻ�ѧ����ʽΪ��_________��
(5)G �Ľṹ��ʽΪ______________��
(6)����˵����ȷ����__________(����ĸ���)��
a����Ϊ������Ӧ
b��B �� D ��Ϊͬϵ��
c��D �ķе��̼ͬԭ������������
d��1mol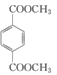 ������ NaOH ��Һ��Ӧʱ��������� 4 mol NaOH
������ NaOH ��Һ��Ӧʱ��������� 4 mol NaOH
(7)J ��ij��ͬ���칹���� J ������ͬ�����ţ���Ϊ˳ʽ�ṹ����ṹ��ʽ�ǣ�______��
(8)д���� PET �����Ʊ� PET ���������� B �Ļ�ѧ����ʽ��__________��
(9)д���ߵĻ�ѧ����ʽΪ��_______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com