
����Ŀ�� A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʡ�����֮�������µķ�Ӧ��ϵ��

(1)��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�������Ҫ�ɷ֣�д���÷�Ӧ�Ļ�ѧ����ʽ��_________________��
(2)��D���ʾ������ԣ��ڡ��۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���塣д����Ӧ�۵����ӷ���ʽ��______________��
(3)��A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A���ڡ��ݷ�Ӧ���õ�ͬһ�ַǽ������ʡ�C����Һ����ʴ��ӡˢͭ��·�壬д���÷�Ӧ�����ӷ���ʽ��______________��
���𰸡�4NH3+6NO![]() 5N2+6H2O Al2O3+2OH-=2AlO2-+H2O 2Fe3++Cu=2Fe2++Cu2+
5N2+6H2O Al2O3+2OH-=2AlO2-+H2O 2Fe3++Cu=2Fe2++Cu2+
��������
(1)��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�������Ҫ�ɷ֣���A��N2��B��NH3��C��NO��D��NO2��
(2)��D���ʾ������ԣ��ڡ��۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���塣��A��Al��B��Al2O3��C��ƫ�����Σ�D��Al(OH) 3��
(3)��A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A���ڡ��ݷ�Ӧ���õ�ͬһ�ַǽ������ʡ�C����Һ����ʴ��ӡˢͭ��·�壬��A��Fe��B��FeCl2��C��FeCl3��D��FeCl2��
(1)��������������֪A��N2��B��NH3��C��NO��D��NO2����B��C��һ�������·�Ӧ���ɵ�A�Ļ�ѧ����ʽΪ��4NH3+6NO![]() 5N2+6H2O��
5N2+6H2O��
(2)��������������֪A��Al��B��Al2O3��C��ƫ�����Σ�D��Al(OH) 3����Ӧ��Al2O3��ǿ����Һ��Ӧ����ƫ�����κ�ˮ����Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
(3)��������������֪A��Fe��B��FeCl2��C��FeCl3��D��FeCl2��FeCl3��Һʴ��ӡˢͭ��·�巴Ӧ�����ӷ���ʽΪ��2Fe3++Cu=2Fe2++Cu2+��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ijԭ��ص�װ��ͼ�����ص��ܷ�Ӧ�ǣ�2Ag��(aq) + Cu(s) �� Cu2��(aq) + 2Ag(s)����ش��������⣺
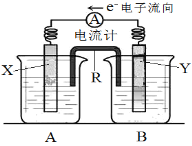
��1��R��������__________��R�е�����������________������A������B�����е���Һ��
��2���缫Y�IJ�����______________��B�еĵ������Һ��________________��
��3��XΪԭ��ص�______��������������������������缫��Ӧʽ��____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ֿ��������쵼�����״��ֺ��Աʹ�õ�����ƿ���Ʊ�����һ���䷽�к��������������ʣ�
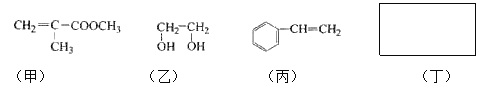
��д���пհף�
��1�����в�����ԭ�ӵĹ�������____________�������Լ������Ӧ����ɫ����___________�����ţ�
a. Br2/CCl4��Һ b.ʯ����Һ c.����KMnO4��Һ
��2����ͬ���칹���ж��֣�д������һ�ֲ�����������Ľṹ��ʽ��_______
��3������ͨ������ת�����Եõ��ң�����A��D��Ϊ�л��:

A�ķ���ʽ��___________���Լ�X������___________��
��4����֪��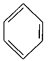 +RCl
+RCl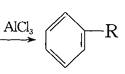 +HCl(-R����)
+HCl(-R����)
 +H2
+H2
����������Ϣ���Ա�����ϩ���Ȼ���Ϊԭ�Ͼ�������Ӧ�ϳɱ�����������ȡ����Ӧ�Ļ�ѧ����ʽ�� ______________ ��
��5�������ﶡ����̼���⡢������Ԫ�أ���Է�������Ϊ110������FeCl3��Һ������������ɫ���Ҷ������������ϵ�һ��ȡ����ֻ��һ�֡��Ľṹ��ʽΪ ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
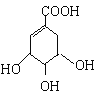
����Ŀ��ç����ϳ����������е�ҩ������ƣ�Tamiflu����ԭ��֮һ��ç������A��һ���칹�塣A�Ľṹ��ʽ���£�
����ʾ��������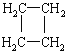 �ɼ�д��
�ɼ�д��![]() ��
��
��1��A�ķ���ʽ��_______
��2��A��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽ���л����ýṹ��ʽ��ʾ��_____
��3��A���Ҵ���Ӧ�Ļ�ѧ����ʽ���л����ýṹ��ʽ��ʾ����_______
��4��17.4g A������̼��������Һ��Ӧ���������ɶ�����̼���������״����____L
��5��A��Ũ���������¼��ȿɵõ�B��B�Ľṹ��ʽΪ![]() �����䷴Ӧ������____��
�����䷴Ӧ������____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ˮ��Һ������ƽ��ԭ���ش��������⣺
I.�����Ļ�ѧʽΪ____��������������ˮ���������ӷ���ʽ��ʾ�����ľ�ˮԭ��____
II.��������Ũ�Ⱦ�Ϊ0.01mol/L��������Һ��HNO3 ��H2S �� NaOH ��NH3��H2O ��NH4Cl ��NH4HSO4 �� NaCl���ش��������⣺
(1)�٢ڢۢ�������Һ����ˮ�������H+��Ũ���ɴ�С��˳��Ϊ______������ű�ʾ����ͬ��
(2)�ܢݢ�������Һ��NH4+Ũ���ɴ�С��˳��Ϊ__________________��
(3)�٢ۢܢݢޢ�������ҺpH�ɴ�С��˳��Ϊ_________________��
(4)������Ģֱܷۢ���ͬŨ�ȵ����ᷴӦ���������ԣ���������������_____�ܣ���������������С������������������
(5)10mL ����20mL�ݻ�Ϻ���Һ��_____�ԣ����������������������������������Һ������Ũ���ɴ�С��˳��Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�������̷�(FeSO4��7H2O)Ϊԭ���Ʊ���Ѫ���ʰ�������[(H2NCH2COO)2Fe]���й�������������:
�ʰ���(H2NCH2COOH) | ������ | �ʰ������� |
������ˮ�������Ҵ������Ի����� | ������ˮ���Ҵ��������Ժͻ�ԭ�� | ������ˮ���������Ҵ� |
ʵ�����:
I.���ƺ�0.10molFeSO4���̷���Һ��
II.�Ʊ�FeCO3:�����ƺõ��̷���Һ�У���������200mL1.1mol/LNH4HCO3��Һ���ӱ߽��裬��Ӧ��������˲�ϴ�ӳ�����
III.�Ʊ�(H2NCH2COO)2Fe:ʵ��װ������ͼ(�гֺͼ���������ʡ��)����ʵ��II�õ��ij����ͺ�0.20mol�ʰ����ˮ��Һ��Ϻ����C�У�Ȼ������A�з�Ӧ���������彫C�п����ž������ŵ�����������Һ�����ȡ���Ӧ��������ˣ���Һ�������ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
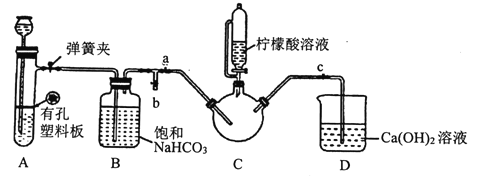
�ش���������:
(1)ʵ��I��:ʵ���������̷���Һʱ��Ϊ��ֹFeSO4���������ʣ�Ӧ������Լ�Ϊ____(д��ѧʽ)��
(2)ʵ��II��:���ɳ��������ӷ���ʽΪ________________��
(3)ʵ��III��:
�ټ��װ��A�������Եķ�����_________��
��װ��A����ʢ�ŵ�ҩƷ��_______ (�����)��
a.Na2CO3��ϡH2SO4 b.CaCO3��ϡH2SO4 c.CaCO3��ϡ����
��ȷ��c�п����ž���ʵ��������______________��
�ܼ�����������Һһ����ɵ�����Һ��pH�ٽ�FeCO3�ܽ⣬��һ��������_______��
��ϴ��ʵ��III�еõ��ij�������ѡ�õ����ϴ���Լ���___(�����)��
a.��ˮ b.�Ҵ���Һ c.��������Һ
������Ʒ������Ϊ17.34g,�����Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�����г��漰��һЩ��Ҫ����ѧ��ѧ��Ӧ�������й�������ȷ����
A.��ҵ�ϣ��ý�̿�ڸ����»�ԭ���������Ƶôֹ�
B.ͨ��������ͨ�뵽����ʯ��ˮ���Ƶô���Ư��
C.��ҵ�����ὫSO2������SO3������һ��ѡ����¡���ѹ������
D.�ƿɰ��ѡ�ﯡ��ꡢ��Ƚ��������ǵ�±������Һ�ﻹԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ƾ���(Na2MoO4.2H2O)��һ����������ȴˮϵͳ�Ľ�����ʴ������ҵ�������⾫��(��Ҫ�ɷ��Dz�����ˮ��MoS2)�Ʊ������Ƶ�����;����ͼ��ʾ��
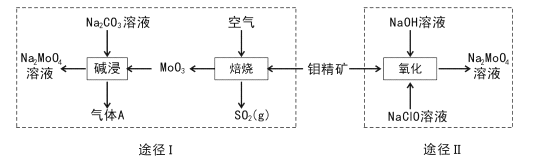
(l) Na2 MoO4![]() 2H2O����Ԫ�صĻ��ϼ�Ϊ____��NaCIO�ĵ���ʽΪ ___��
2H2O����Ԫ�صĻ��ϼ�Ϊ____��NaCIO�ĵ���ʽΪ ___��
(2);��IΪ������⾫�������ʣ��ɲ�ȡ�Ĵ�ʩ��____(�����������)��
(3);��I���չ����л�ѧ����ʽΪ____�����ʱ����A�Ļ�ѧʽΪ____��
(4)��֪;��I����������Һ��c(MoO42-)=0. 40mol/L��c(CO32-)=0. 20mol/L������������Һ�Ʊ������ƾ���ʱ�������Ba(OH)2�����Գ�ȥCO32-����BaMoO4��ʼ����ʱ��CO32-��ȥ������____(��֪Ksp(BaCO3) =1��10-9��Ksp(BaMo04) =4.0��10-8��������Һ������仯)��
(5);��II����ʱ��Һ�л���Na2SO4���ɣ���ԭ�������������ʵ���֮��Ϊ_____��
(6);��I��;��II���õ�Na2MoO4��Һ���ᾧ�ɵù���A�����ؽᾧ�ɵù���B�����ؽᾧ������Ŀ��Ϊ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʹ���ʸ������������(Na2SiF6)Ϊԭ�Ϻϳɱ���ʯ(Na3AlF6)��Ϊһ������������Դ����߾���Ч����·�����������������ͼ��ʾ��
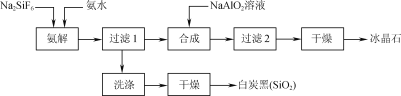
��1����ͳ�ϳɱ���ʯ�ķ�����өʯ(CaF2)����ʹ��өʯ��ʯӢ�ʹ����ڸ�������������NaF����NaF��Һ�м���Al2(SO4)3��Һ�Ƶá��ڼ�����������Һǰ�����������ὫNaF��Һ��pH�µ���5���ң�
������ܲ���������____(�����ʵĻ�ѧʽ)������Ȳ��˹�ǿ��ԭ����____��
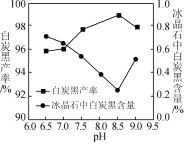
��2������ʱ��̿�ڲ��ʺͱ���ʯ������pH�Ĺ�ϵ��ͼ������ʱ��Ҫ������Һ��pH��____������߰������ʵĴ�ʩ��____(����ĸ)��
A�����ٽ���
B�����Ȼ��Һ��100��
C����С��ˮŨ��
��3�����������а��ⷴӦ�Ļ�ѧ����ʽΪ____������ʯ�ϳɷ�Ӧ�����ӷ���ʽΪ______��
��4��Ϊ�����ԭ�������ʣ����ٻ�����Ⱦ���ɲ�ȡ�Ĵ�ʩ��___��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com