
����Ŀ��A��B��C��D��E��Ϊ�����ڵ�����Ԫ�أ���ԭ��������������A��ԭ�ӵ��Ӳ�������������������B��C���γ����ӻ�����CB2��Dԭ�ӵ�M���������K���������3����
(1)д��A�ļ������ӵĵ���ʽ��________��
(2)E���⻯����C������ȣ��е�ϵ͵���_________(�ѧʽ)��
(3)C��E��ɵĻ�����������ѧ����������______________��
(4)B��D��E��ԭ�Ӱ뾶�ɴ�С��˳������Ϊ_____________(��Ԫ�ط���)��
(5)E������������Ӧ��ˮ���ﻯѧʽ��____________
(6)��D��E����ɻ�����D2E2 ���ڸû������и�ԭ���������ﵽ8�����ȶ��ṹ��д����ṹʽ__________________
(7)д��E��Ԫ�����ڱ��е�λ��____________________________________��
(8)��D����ۺ������Ũ��Һ¶�ÿ����У������ᷢ���仯�����������Ӧ���_____________��
���𰸡�![]() HCl ���Ӽ� S>Cl>F HClO4 Cl-S-S-Cl ��������VII A�� ��ˮ
HCl ���Ӽ� S>Cl>F HClO4 Cl-S-S-Cl ��������VII A�� ��ˮ
��������
A��B��C��D��E��Ϊ�����ڵ�����Ԫ�أ���ԭ��������������A��ԭ�ӵ��Ӳ���������������������AΪHԪ�أ�D��ԭ��M���������K���������3��������M�������Ϊ6����DΪSԪ�أ�E��ԭ���������EΪClԪ�أ�B��C���γ����ӻ�����CB2����C����+2�ۡ�B����-1�ۣ�����ԭ������С������BΪFԪ�ء�CΪMg��
��������������֪AΪHԪ�أ�BΪFԪ�أ�CΪMgԪ�أ�DΪSԪ�أ�EΪClԪ�ء�
(1)A��Ԫ�ط�����H��A�ļ������ӵĵ���ʽ��![]() ��
��
(2)E��Cl��E���⻯��HCl���������ڳ�����Ϊ��̬��C����ΪMg����������Ϊ��̬���������ʵķе�ϵ͵���HCl��
(3)C��E��ɵĻ�����ΪMgCl2���û�����Ϊ���ӻ����Mg2+��2��Cl-ͨ�����Ӽ���ϣ��������������ѧ�������������Ӽ���
(4) BΪFԪ�أ�DΪSԪ�أ�EΪClԪ�أ�ͬһ���ڵ�Ԫ�أ�����ԭ������������ԭ�Ӱ뾶��С��ͬһ����Ԫ�أ�����ԭ������������ԭ�Ӱ뾶�������������Ԫ�ص�ԭ�Ӱ뾶��S��Cl��F��
(5)EΪClԪ�أ�E������������Ӧ��ˮ���ﻯѧʽ��HClO4��
(6)��D��E����ɻ�����D2E2 ��S2Cl2���ڸû������У�2��Sԭ��֮���γ�1�Թ��õ��Ӷԣ�ÿ��Sԭ����1��Clԭ���γ�1�Թ��õ��Ӷԣ������и�ԭ���������ﵽ8�����ȶ��ṹ����ṹʽΪCl-S-S-Cl��
(7) EΪClԪ�أ���������Ų���2��8��7������E��Ԫ�����ڱ��е�λ����λ�ڵ������ڵ�VIIA�塣
(8)DΪS����D����ۺ������Ũ��Һ��ΪŨ���ᣬ�����ʾ�����ˮ�ԣ�¶�ÿ����У������տ����е�ˮ������ʹ��Һ���������ӣ������������Ӧ�����ˮ�ԡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ��Ⱦ��Ҫ���� ����Ȼˮ���������ʯ���������ڽӴ� ����ҵ�����з�������Һ���������ŷ� ��ˮ������ķ�ֳ ������������ˮ�Ĵ����ŷ� ��ũҵ������ũҩ������ʹ�ò���
A�� �ܢ� B���٢ڢ� C ���ڢܢ� D�� �ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.�ں��������½�һ����X��Y�Ļ������ͨ��һ�ݻ�Ϊ2 L���ܱ������У�X��Y�����ʵ�Ũ����ʱ��仯�������ͼ��

(1)�÷�Ӧ�Ļ�ѧ����ʽΪ(��Ӧ����������÷���X��Y��ʾ)�� _______________��
(2)a��b��c��d�ĸ����У���ʾ��ѧ��Ӧ����ƽ��״̬�ĵ���_________��
��.��ͼ�ǿ��淴ӦX2��3Y2![]() 2Z�ڷ�Ӧ�����еķ�Ӧ����(v)��ʱ��(t)�Ĺ�ϵ���ߣ�����������ȷ����________
2Z�ڷ�Ӧ�����еķ�Ӧ����(v)��ʱ��(t)�Ĺ�ϵ���ߣ�����������ȷ����________

A.t1ʱ��ֻ��������Ӧ B.t2ʱ����Ӧ�ﵽ��
C.t2��t3����Ӧ���ٷ��� D.t2��t3�������ʵ�Ũ�Ȳ��ٷ����仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ba(OH)2(����)��CuSO4(����)����H2SO4(Һ̬)��Ϊһ�࣬�����������ʿ��Ժ����ǹ�Ϊһ�ࣨ ��
A.75%�ľƾ���ҺB.�Ȼ���C.ϡ����D.�ཬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ʳƷ��ҵ����������ķ�ɫ������һ���ĵ������ԣ��������Ʊ�¶�ڿ����л���������Ӧ���������ƣ�һ��ģ�ҵ�ô�����Һ���յ��������Ʊ�NaNO2��ʵ��װ�����£�

��֪��NO2+NO+2NaOH=2NaNO2+H2O��2NO2+2NaOH=NaNO2+NaNO3+H2O
�ش��������⣺
(1)������ӦʱNO��NO2�����ʵ���֮��Ϊ1��1ʱ��A�з�����Ӧ�Ļ�ѧ����ʽΪ________��
(2)װ��B��������____________________��װ��C��ͨ��O2��Ŀ����_______________��
(3)��Ӧ��A����Һ������Ũ�����ᾧ������õ���Ʒ��
��ͬѧȡ�����IJ�Ʒ����ˮ����ϡ�����ữ����ʪ���KI������ֽ���飬��ֽ�������ó���ƷΪNaNO2����ͬѧ��Ϊ���۲��ɿ�����������____________________________��
(4)����V LijNaOH��Һ����ȫ����n mol NO2�� m mol NO��ɵĴ�����Ⱦ�
�������ռ���Һ�����ʵ���Ũ������Ϊ______________ mol/L��
����������Һ��c(NO3-)��c(NO2-)=1��9����ԭ���������NO2��NO�����ʵ���֮��n��m=________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ�
A. 28g����ϩ�к���NA��̼̼˫��
B. 1mol��ϩ��Cl2��ȫ�ӳɣ�Ȼ����Cl2����ȡ����Ӧ�������������ķ��������Ϊ6NA
C. ��״���£�2.24LCCl4�е�ԭ����������0.5NA
D. 15g�����еĵ�������9NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(Na2S2O3)�����������о��й㷺Ӧ�á��ش��������⣺
I.��ҵ���ձ�ʹ��Na2SO3����ǹ����Ʊ�Na2S2O3��װ����ͼ1��

��1����K1�ر�K2����Բ����ƿ�м��������Լ��ײ����ȡ��Լ���Ϊ_________��װ��B��D��������_________��
��2��ʼ�ձ���C����Һ�ʼ��ԡ����Ȳ���Na2S2O3����Ӧ�Ļ�ѧ����ʽΪ___________________________����Ӧһ��ʱ���C��������٣���ʱ��K2���ر�K1��ֹͣ���ȣ���C�����û��������ᴿ�õ�Na2S2O3��������ʱ�ر�K1��������C����Һ�����ԡ���������Ӧ����S��_________��
��.����SO2��Na2CO3��Na2S�Ļ����Һ��ӦҲ���Ʊ�Na2S2O3������������ͼ2��

��1��װ��G��Na2CO3��Na2S��������ʵ���֮��Ϊ_________��
��2�����������Ӹ��������ӿ�˳��Ϊ��_________��g��h��_________��_________��_________��_________��d��
��.����Na2S2O3��Һ�ⶨ��ˮ��Ba2+Ũ�ȡ�
ȡ��ˮ20.00mL�������ʵ�����ȼ�������K2Cr2O7��Һ���� BaCrO4����������ϴ�Ӻ�������ϡ���ܽ⣬��ʱCrO42��ȫ��ת��ΪCr2O72�����ټӹ���KI��Һ����Cr2O72����ַ�Ӧ��Cr2O72��+6I��+14H+=3I2+2Cr3++7H2O��Ȼ����������Һ��ָʾ������0.0100mol/L��Na2S2O3��Һ���еζ���I2+2S2O32��===S4O62��+2I��������Һ_________��Ϊ�յ㡣ƽ�еζ�3�Σ�����Na2S2O3��Һ��ƽ������Ϊ18.00m����÷�ˮ��Ba2+�����ʵ���Ũ��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ƚ�1mol������1molһ����̼�����������������������ڷ�����������ԭ��������������ͬ���ǣ� ��
A.��B.�٢�C.�ڢ�D.�٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�ַdz���Ҫ�Ľ���Ԫ�أ��ںܶ��������Ź㷺��Ӧ�á����÷���м��ԭ������Ʒλ���̿��Ʊ������̣�Ȼ����е�⣬���Ʊ������̵��¹��գ������̼�ͼ���£�
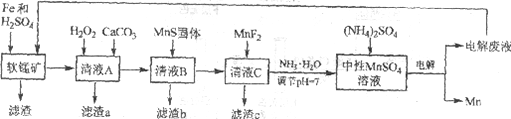
��֪��
i. ��Ʒλ���̿���Ҫ�ɷ���MnO2��Al2O3��Fe2O3��Cu2(OH)2CO3��CaCO3��SiO2�ȡ�
ii. ���ֽ��������������������ʱ��pH
Fe2+ | Fe3+ | Al3+ | Mn2+ | Cu2+ | |
��ʼ������pH | 6.8 | 1.8 | 3.7 | 8.6 | 5.2 |
������ȫ��pH | 8.3 | 2.8 | 4.7 | 10.1 | 6.7 |
iii. ���ֻ�������ܽ��Ի��ܶȻ�(Ksp)
MnF2 | CaS | MnS | FeS | CuS |
����ˮ | ����ˮ | 2.5��10��13 | 6.3��10��18 | 6.3��10��36 |
(1)�о�����������Fe��Fe2+�����Ի�ԭMnO2����������ڵ������£�MnO2��Fe����Ϊspan>Fe3+�����ӷ���ʽ��____________��
(2)��ҺA����H2O2������Ȼ�����CaCO3����Ӧ����Һ��pH��5������a����Ҫ�ɷ����л������NH4Fe3(SO4)2(OH)6��
��H2O2��������____________(�����ӷ���ʽ��ʾ)��
������a�г��˻����������Ҫ�ɷֻ���X����ƽ���ƶ�ԭ�����Ͳ���x��ԭ��____________��
(3)�����ӷ���ʽ��ʾMnS��������ã�____________��
(4)����c�ijɷ���____________��
(5)����ͼ��ʾװ�ã��ö��Ե缫�������MnSO4��Һ�����Ƶý���Mn��������Ӧ�У�
i. Mn2++2e����Mn ii. 2H++2e����H2��
�缫��H2�IJ�������������ǿ��ѣ�Ӱ���Ʒ������
�ٵ��������ĵ缫����ʽ��________________��
����ҺC��Ҫ�ð�ˮ����pH��7��ԭ����____________��
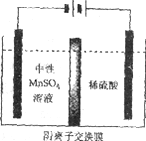
�۵��ʱ������MnSO4��Һ�м���(NH4)2SO4�����ó���������Һ������֮�⣬����___________(��ϵ缫��Ӧʽ�����ӷ���ʽ����)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com