
����Ŀ���밴Ҫ��������и��⣺
(1)��������50 g�� HCl��NH3��CO2��O2���������У����з�����Ŀ���ٵ���__________
(2)ij��Һ��ֻ����Na+��Al3+��Cl����SO42-�������ӣ���֪ǰ�������ӵĸ�����Ϊ1��2��1�� ����Һ��Al3+��SO42-�����Ӹ�����Ϊ__________��
(3)��һС����Ͷ�뵽ʢCuSO4��Һ���ձ��У����ҷ�Ӧ���ų����岢������ɫ���������ܷ�Ӧ�����ӷ���ʽΪ________________��
(4)��FeSO4��Һ�����NaOH��Һ��ϲ��ڿ����з���һ��ʱ�䣬���������е�����Ϊ______����Ӧ���̷����������е�2����Ӧ�Ļ�ѧ����ʽΪ__________��
���𰸡�CO2 2��3 2Na��2H2O��Cu2+=2Na+��Cu(OH)2����H2�� ���ɰ�ɫ��״������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ 4Fe(OH)2��O2��2H2O=4Fe(OH)3
��������
��1��HCl��NH3��CO2��O2����������CO2����Է������������n=![]() ��֪���������Է�������Խ��Ħ������Խ������ʱ��������ʵ���ԽС��Ħ�������ɴ�С��˳��Ϊ��CO2>HCl>O2>NH3�����������С����CO2��
��֪���������Է�������Խ��Ħ������Խ������ʱ��������ʵ���ԽС��Ħ�������ɴ�С��˳��Ϊ��CO2>HCl>O2>NH3�����������С����CO2��
�ʴ�Ϊ��CO2��
��2����Һ���ڵ���غ㣬Ϊn��Na+��+3n��Al3+��=n��Cl-��+2n��SO42-������֪ǰ�������ӵĸ�����Ϊ1��2��1�������Ϊ1mol��2mol��1mol����n��SO42-��=3mol������Һ��Al3+�� SO42-���Ӹ�����Ϊ2��3��
�ʴ�Ϊ��2��3��
��3����һС����Ͷ�뵽ʢCuSO4��Һ���ձ��У����ҷ�Ӧ��Na��ˮ��Ӧ�����������ƺ�����������������Һ������ͭ��Һ��Ӧ����������ͭ�����������ƣ��ʷ�Ӧ�������ӷ���ʽ�ǣ�2Na��2H2O��Cu2+=2Na+��Cu(OH)2����H2����
�ʴ�Ϊ: 2Na��2H2O��Cu2+=2Na+��Cu(OH)2����H2��
(4) ��FeSO4��Һ�����NaOH��Һ������������������������ƣ������������ڿ����в��ȶ��������������������������ʿ��������������ɰ�ɫ��״������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ����Ӧ���̷����������е�2����Ӧ�Ļ�ѧ����ʽΪ4Fe(OH)2��O2��2H2O=4Fe(OH)3��
�ʴ�Ϊ�����ɰ�ɫ��״������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��4Fe(OH)2��O2��2H2O=4Fe(OH)3



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺
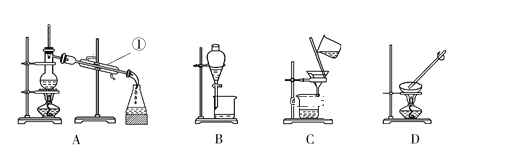
(1)���Ȼ�����Һ�еõ��Ȼ��ع��壬ѡ��װ��________(�����װ��ͼ����ĸ����ͬ)�����뱥��ʳ��ˮ��ɳ�ӵĻ���ѡ��װ��________��
(2)�ӵ�ˮ�з����I2��ѡ��װ��________���÷��뷽��������Ϊ________��
(3)װ��A�Тٵ�������______����ˮ�ķ����Ǵ�____��(����������������)��ˮ��װ��B�ڷ�ҺʱΪʹҺ��˳�����£�����©���¶˵������⣬��Ӧ���еľ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)25��ʱ����ͬ���ʵ���Ũ�ȵ�������Һ����NaCl��NaOH��H2SO4��(NH4)2SO4������ˮ�ĵ���̶Ȱ��ɴ�С˳������Ϊ___________(������)��
(2)��25���£���amol��L��1�İ�ˮ��0.01 mol��L��1������������ϣ���Ӧƽ��ʱ��Һ��c(NH4+)=c(Cl��)������Һ��___________(��ᡱ�����)�ԣ��ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb=___________��
(3)һ���¶��£���ˮ��ͨ��CO2���õ���NH4)2CO3��NH4HCO3�����ʣ���Һ�и����������ʵ���������pH�Ĺ�ϵ��ͼ��ʾ������CO2��ͨ�룬��Һ��c(OH��)/c(NH3��H2O)��___________(�������С�����䡱)��pH=9ʱ����Һ��c(NH4+)+c(H+)=___________��

(4)����������(Na2S2O5)������ʳƷ�Ŀ������������������Ѿơ�������ʳƷ�С��ڲⶨij���Ѿ���Na2S2O5������ʱ��ȡ25.00mL���Ѿ���Ʒ����0.01000mol��L��1�ĵ��Һ�ζ����յ㣬����5.00mL���õζ���Ӧ�����ӷ���ʽΪ___________�����Ѿ��е�Na2S2O5��ʹ��������SO2������ģ������Ʒ��Na2S2O5�IJ�����Ϊ___________g��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����л�� ��CH3��CH2��CH2��CH3�� ![]() ��CH2=CH��CH2��CH3��CH3�� CH=CH��CH3
��CH2=CH��CH2��CH3��CH3�� CH=CH��CH3
��CH3��CH2��OH��CH3��O��CH3�� ��
�� ��CH3��CH2��CH=CH��CH3��
��CH3��CH2��CH=CH��CH3�� ![]() ��CH2=CH
��CH2=CH
��CH=CH2��CH3��CH2��C��CH��
(1)��������ͬ���칹�����________________��
(2)��������̼���칹�����________________��
(3)�������ڹ�����λ���칹����________________��
(4)�������ڹ����������칹����________________��
(5)��������ͬһ�����ʵ���________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ����Ҫ�ɷ��Ƕ�������ͭ��CuFeS2������ͭ�����������պ�õ���ͭ��¯����ұ�����̵���Ҫ��Ӧ�У�

��1����������ͭҲ���Ա�ʾΪCuS��FeS��������Ԫ�صĻ��ϼ���____��
��2����Ӧ���л�ԭ����________��
��3��ijУѧϰС������ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�ȣ��Ʊ����죬��������ʵ�顣
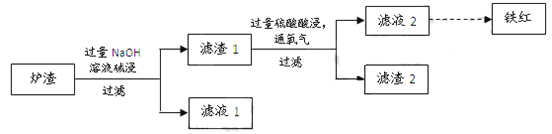
�� ¯�����ʱ��Ӧ�����ӷ���ʽ��_____��_____��
�� ����1�м������Ტͨ��������ʹFeOת��ΪFe3+���÷�Ӧ�����ӷ���ʽ��________��Ϊ������Ԫ���Ƿ�������ȫ��Ӧ���е�ʵ���ǣ�ȡ������Һ2���Թ���_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����¶�t1��t2�£�X2��g����H2��Ӧ����HX��ƽ�ⳣ�����±���
��ѧ����ʽ | K��t1�� | K��t2�� |
F2+H2 | 1.8��1036 | 1.9��1032 |
Cl2+H2 | 9.7��1012 | 4.2��1011 |
Br2+H2 | 5.6��107 | 9.3��106 |
I2+H2 | 43 | 34 |
��1����֪t2��t1��HX�����ɷ�Ӧ��___��Ӧ������ȡ����ȡ�����
��2��HX�ĵ���ʽ��___��
��3�����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ��HX���ۼ��ļ�����ǿ������˳����___��
��4��X2������H2��Ӧ����HX����ԭ�ӽṹ����ԭ��___��
��5��K�ı仯���ֳ�X2��ѧ���ʵĵݱ��ԣ���ԭ�ӽṹ����ԭ��___��ԭ�Ӱ뾶�����õ�������������
��6��������K�ı仯�������ƶϳ�������±��ԭ�Ӻ˵���������ӣ�___��ѡ����ĸ����
a������ͬ�����£�ƽ��ʱX2��ת��������
b��X2��H2��Ӧ�ľ��ҳ̶�����
c��HX�Ļ�ԭ������
d��HX���ȶ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״����CH4��H2S��NH3��Ϊ���壬�ֱ�����11.2L H2S��16g CH4��1.204��1024��NH3���ӣ�������������С�Ƚ���ȷ���ǣ� ��
A. ���������������
B. �ܶȣ�����������
C. ����������������
D. ԭ������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ʊ��治�����ױ��ʣ�ij����С��Ϊ�˴��Բⶨ�������Ƶ��������������dz�ȡ10.0g��Ʒ�����������ͼװ�����ⶨ�������Ƶ�����������

��ͼ�е�E��F��������װ�ã������ⶨO2�������
��1��д������װ�÷�����Ӧ�Ļ�ѧ����ʽ��
װ��A��___________________________��
װ��B��___________________________��
װ��C��____________________________��
��2��NaOH��Һ��������_________________________��
��3��Ϊȷ������������������²�������ȷ��˳��Ϊ_________��
A��������Ͳ�߶ȣ�ʹ���ƿE����ͲF��Һ����ƽ
B����������ȴ������
C��ƽ�ӣ�ʹ��Һ����͵�������ˮƽ�����ٶ���
������Ͳ��ˮ�����������ɱ�״�����������Ϊ1.12L������Ʒ�й������Ƶ���������Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧ����ѡ��3�����ʽṹ�����ʣ�
��23��Ԫ�ط��ڵؿ��еĺ�����ԼΪ0.009%���ڹ���Ԫ���н�����Fe��Ti��Mn��Zn���ŵ���λ���ҹ��Ĵ���֦�������̲��ż���ḻ�ķ��Ѵ�����
(1)�������ڱ��е�λ��Ϊ__________������ռ�ݵ�����ܲ�Ĺ����״Ϊ_______
(2)�ڵؿ��к�����ߵ����ֹ��ɽ���Ԫ��Fe��Ti��Mn��Zn��V�У���̬ԭ�Ӻ��ⵥ������������_____��
(3)���ɽ������γ������Ȼ�������CO��Ϊ�����γɵ�����
��CO�ĵȵ�������N2��CN����_______��(��дһ��)��
��CO������ʱ����λԭ����C������O����ԭ����________��
(4)���ɽ�������ﳣ������18���ӹ�������������ԭ�ӵļ۵��������������ṩ�ĵ�����֮�͵���18����[Fe(CO)5]��[Mn(CO)5]���ȶ������������
�����з�������У���ԭ������18���ӹ������__________��
A [V(H2O)6]2�� B [V(CN)6]4�� C [V(CO)6]�� D [V(O2)4]3��
�ڻ����� ���۵�Ϊ138�棬�侧������Ϊ________��
���۵�Ϊ138�棬�侧������Ϊ________��
(5)VCl2(�۵�1027��)��VBr2(�۵�827��)��Ϊ�����������ṹ��ͼ��ʾ��

��VCl2��VBr2�����۵�����ԭ����_________��
���辧�������������Ӱ뾶�ֱ�Ϊr����r�����þ���Ŀռ�������Ϊ________(�ú�a��c��r����r����ʽ�ӱ�ʾ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com