
����Ŀ����������������Ԫ�أ���ҽҩ��ҵ�ж��й㷺��;����˴Ӻ����Һ�л��յ������ö�����Դ�Ƿdz���Ҫ�ġ�ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I-���л��յ⣬��ʵ�������ͼ1������֪������Cl2> IO3-��
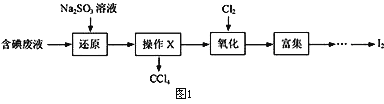
��1�����Һ�м����Թ�����Na2SO3��Һ����Ӧ�����ӷ���ʽΪ_________���ò�����I2��ԭΪI-��Ŀ����_________________________________________��
��2������X������Ϊ______________________________________��
��3������ʱ����������ƿ�н���I-��ˮ��Һ���������pHԼΪ2��ͨ��Cl2����40�����ҷ�Ӧ��ʵ��װ����ͼ2��ʾ���������й�˵����ȷ����_____������ĸ����
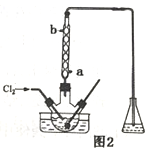
a.��ʵ����Ҫ����ͨ����������������������ʣ���ֹ������Ⱦ
b.�����ڽϵ��¶��½��е���Ҫԭ���������������ܽ��
c.ͨ���������������ߵ�IJ���
d.Ϊ��Ч��ֹ��Ļӷ�ˮӦ��b��ͨ��
e.��ƿ��Ӧʢ��NaOH��Һ
��4���������ȣ�C1O2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ�����������ˮ������������C1O2�������Ժ�I-��Һ���յ⡣
��д��C1O2����I-�����ӷ���ʽ____________________________________��
����������I-��ͬ���ķ�Һ���յ⡢����Cl2�����ʵ�����C1O2��______________����
��5����֪��5SO32-+2IO3-+2H+![]() I2+5SO42-+H2O��ij�����ˮ��pHԼΪ8����һ������I2�����ܴ���I-��IO3-�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I-��IO3-��ʵ�鷽������ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
I2+5SO42-+H2O��ij�����ˮ��pHԼΪ8����һ������I2�����ܴ���I-��IO3-�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I-��IO3-��ʵ�鷽������ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
��ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ����鲻���ⵥ�ʴ��ڣ�
��_______________________��
������ˮ��ȡ������Һ������2-3�ε�����Һ���μ�________________�����Լ�������˵����ˮ�к���IO3-������˵����ˮ�в���IO3-��
���𰸡� H2O+SO32-+I2=2I-+SO42-+2H+ ʹCCl4�еĵ����ˮ�㣨��I2ת��ΪI-��CCl4�з�������� ��Һ abe 2C1O2+10I-+8H+=2Cl-+5I2+4H2O 2.5 ��ˮ����ȡ������Һ������2~3�ε�����Һ����ϡ�����ữ���μ�FeCl3��Һ������Һ��������֤����ˮ�д���I-������˵����ˮ�в���I- ����ϡ���������������Һ������Һ����
��������I�������Һ(��H2O�⣬����CCl4��I2��I-)�м�����������Һ���ѵ��ʵԭΪI-�����Ȼ�̼������ˮ����ֲ㣬���Һ���ɵõ����Ȼ�̼��ʣ�����Һ�м���������I-�õ�I2�����������õ��ϴ���I2��
(1)����������ԣ������������������������ƣ���������ԭ���ɵ����ӣ����ӷ�Ӧ����ʽΪSO32-+I2+H2O=2I-+2H++SO42-����������ˮ����������������ˮ��Ϊ��ʹ�����IԪ�ؽ���ˮ��ҺӦ���ԭΪ�����ӣ��ʴ�Ϊ��SO32-+I2+H2O=2I-+2H++SO42-��ʹCCl4�еĵ����ˮ��(��I2ת��ΪI-��CCl4�з������)��
(2)���Ȼ�̼������ˮ������ֲ㣬���뻥�����ܵ�Һ����÷�Һ�ķ������룬���Է�������Ȼ�̼���÷�Һ�ķ������ʴ�Ϊ����Һ��
(3)a.��ʵ����Ҫ����ͨ��������ʹ������ַ�Ӧ��������������������ʣ���ֹ������Ⱦ����ȷ��b.������ܽ�����¶ȵ����߶����ͣ������ڽϵ��¶��½��п��������������ܽ�ȣ���ȷ��c.ͨ���������������֤��������ȫ���������������������ܹ���������������ɺ���Ԫ�ص������ӣ��������͵�IJ���������d.��ȴˮӦ����ѭ�½��ϳ���Ϊ��Ч��ֹ��Ļӷ�ˮӦ��a��ͨ�룬����e.Ϊ�˷�ֹ������Ⱦ��������ƿ��Ӧʢ��NaOH��Һ��������������������ȷ����ѡabe��
(4)����ClO2�������Ժ�I-��Һ���յ⣬�Ƕ�������������Һ���������������ɵⵥ�ʣ��������ȱ���ԭΪ�����ӣ�ClO2��Cl-��5e-��2I-��I2��2e-����Ӧ�����ӷ���ʽΪ2ClO2+10I-+8H+=5I2+2Cl-+4H2O���ʴ�Ϊ��2ClO2+10I-+8H+=5I2+2Cl-+4H2O��
����������ԭ��Ӧ�����غ㣬ÿĦ��Cl2�õ�2mol���ӣ���ÿĦ��ClO2�õ�5mol���ӣ�������Cl2�����ʵ�����ClO2��2.5�����ʴ�Ϊ��2.5��
(5)�ڵ����Ӿ��л�ԭ�ԣ��ܱ��������������ɵ⣬��������Ӿ��������ԣ��ܱ���ԭ����ԭ���ɵ⣬����������Һ����ɫ����������鷽��Ϊ����ˮ��ȡ������Һ������1-2mL������Һ�����������ữ���μ�FeCl3��Һ��2I-+2Fe3+=2Fe2++I2������Һ����ɫ��˵����ˮ�к���I-������I-���ʴ�Ϊ����ˮ��ȡ������Һ������1-2mL������Һ�����������ữ���μ�FeCl3��Һ������Һ����ɫ��˵����ˮ�к���I-������I-��
������ˮ��ȡ������Һ������2-3�ε�����Һ���μ�����ϡ���������������Һ������Һ������˵����ˮ�к���IO3-������˵����ˮ�в���IO3-���ʴ�Ϊ������ϡ���������������Һ������Һ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��±���������þ����ˮ�����з�Ӧ���ɵø����Լ�R��MgX��������ȩ��ͪ���ʻ�������ӳɣ�![]() ���ò��ᆳˮ����Եõ���������ijЩ���Ӵ��ĺϳɷ���֮һ�������ϳ�(CH3)3C-OH��������ѡ�õ�±�������ʻ�������������ȷ����
���ò��ᆳˮ����Եõ���������ijЩ���Ӵ��ĺϳɷ���֮һ�������ϳ�(CH3)3C-OH��������ѡ�õ�±�������ʻ�������������ȷ����
A. ��ȩ�������� B. ��ȩ��1-�����
C. ��ͪ��һ�ȼ��� D. ��ȩ��2-�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������(Na2S2O5)Ϊ��ɫ���ɫ�ᾧ��ĩ��С�ᾧ�������ʻ��ã�����ǿ��ԭ�ԣ��dz��õ�ʳƷ��������֮һ���Ʊ������ʵķ�Ӧ����ʽΪ�� Na2SO3+SO2= Na2S2O5��ij�о�С�����ø÷�Ӧ��ʵ�����Ʊ����������Ʋ�̽�����й����ʡ���ش��������⣺
��1��������ͼװ����ȡNa2S2O5��װ�â�����Na2S2O5����������
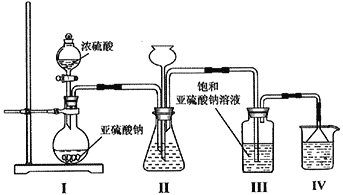
��װ�â���ʢ��Ũ�������������Ϊ__________���μ�Ũ����ǰ��Ҫ��װ�ÃȵĿ����ž�������������Ŀ����___________________________________��
��װ�â������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ��____________________��
a.����ˮ b.����Na2SO3��Һ c.����NaHSO3��Һ d.����NaHCO3��Һ
�۴�װ�â��з����Na2S2O5����ɲ�ȡ�IJ���������_______________���b�â���������_________________________��ʢװ���Լ�Ϊ_____________________��
��2����0.5mol Na2S2O5����ˮ���1L��Һ����ø���ҺpH=4.5����Һ�в�����Ũ������Һ����Ա仯�������ͼ��ʾ��
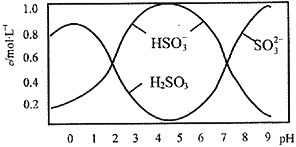
��д������������Na2S2O5�ܽ���ˮʱ��ˮ��Ӧ�Ļ�ѧ����ʽ_____________________��
�ڽ����Һ�е�����ƽ���֪ʶ������Һ�����Ե�ԭ��_____________________________��
��3������Na2S2O5�����ڿ������ѱ�������ʵ�鷽����_________________________��
��4�����ѾƳ���Na2S2O5���������������Ѿ��п��������IJ�����ͨ����������SO2�ĺ������㣬�ҹ����ұ�(GB2760��2014) �涨���Ѿ���SO2�IJ�������0.25g/L�����о�С��ⶨij���Ѿ��п��������IJ�����(������SO2����) �ķ������£�
![]()
����֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2+I2+2H2O=H2SO4+2HI��
��������������ʵ�飬���ı�I2��Һ20.00mL���ô�ʵ������Ʒ�п��������IJ�����Ϊ___g��L-1��������˵��ij���Ѿ���SO2�IJ�����_________ ����ǡ����ﵽ�ҹ����ұ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��ʹAlCl3��Һ�е�Al3+���Ӿ�����ȫ��ת��ΪAl��OH��3����������ʵ��Լ��ǣ� ��
A.NaOH��Һ
B.KOH��Һ
C.����
D.��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���Ũ�Ⱦ�Ϊ0.1 molL-1�������Ϊ100mL������һԪ��HX��HY����Һ�У��ֱ����NaOH ���壬lg![]() �����NaOH�����ʵ����ı仯��ͼ��ʾ�����Լ���NaOH���嵼����Һ�¶ȵı仯��������������ȷ����.
�����NaOH�����ʵ����ı仯��ͼ��ʾ�����Լ���NaOH���嵼����Һ�¶ȵı仯��������������ȷ����.
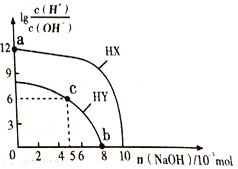
A. HX����������HY
B. c����Һ��c(Y-)<c(HY)
C. a����ˮ�������c(H+)=10-12 molL-1
D. b����Һ������Ũ�ȴ�С��ϵΪc(Y-)+c(HY)=c(Na+)+0.02 molL-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���㷺���ڷ�֯��ҵ��������������(Na2S2O4)���׳Ʊ��շۣ���һ��ǿ��ԭ����������ˮ�����������Ҵ����ڼ��Խ������ȶ���
��.��ҵ���Ʊ������������Ƶ��������£�

��ش��������⣺
��1��������еĻ�ѧ����ʽΪ___________________________________��
��2��������г���Ϊ_________________________________���ѧʽ����
��3��������м���NaCl �����������______________________________����������շ۷���Ϊ_______��ϴ�ӡ����ϴ�������Լ���___________________________��
��.��ҵ��Ҳ������ͼװ�õ��NaHSO3��Һ��Na2S2O4��
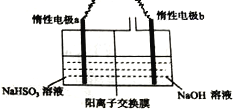
��1�����Ե缫a���ӵ�Դ��_________���������������������缫��ӦʽΪ________________��
��2�������Ӹ�Ĥ����ò��������������ƣ���ԭ����____________________________��
��.̽��Na2S2O4�����ʣ�
ij����С�鳣���²ⶨ0.050 molL-1Na2S2O4��Һ�ڿ����е�pH�仯����ͼ��ʾ��
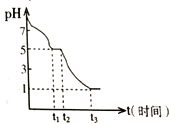
��1��0-t1����Ҫ����HSO3-������pH�仯ͼ��д��0- t1������Ӧ�����ӷ���ʽΪ______________��
��2����t1ʱ��Һ��Na2S2O4ȫ����������NaHSO3����ʱ��Һ��c(SO32-)-c(H2SO3) =__________ molL-1���������ֵ����������Һ����仯��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���ڳ����£���(C6H6)Ϊһ����ɫ������Һ�壬���Խϸߣ��ӷ�����ȼ���и�ʴ�ԣ��е�Ϊ80.1�棬������ˮ���������л��ܼ�������Ϊ�л��ܼ�����(I2)Ϊ�Ϻ�ɫ���壬�����������������۵�113.5�棬�е�184.3�棬����ʱ������Ϊ��ɫ��������ȴ���������Ϻ�ɫ���塣ʵ���ҳ��ñ�����ȡ��ˮ�еĵ⣬����������£�

������ˮ��Һ�м�����ȡ������ת�Ƶ���Һ©���У����ϲ����������������(��ͼ1)��
�ڽ���Һ©����������̨����Ȧ�Ͼ���(��ͼ2)��
�۵���Һ©���е�Һ��ֳ�����������������ʵIJ�����������Һ����з��룻
�ܽ�������ĵ�ͱ��Ļ��Һת�Ƶ�����A�У�����������ʯ���������Է��뱽�͵�(��ͼ3)��
��ش��������⣺
��1����Һ©����ʹ��ǰ������еIJ�����_______��
��2��������С����ʵIJ�������������________��
��3��������в���ˮԡ���ȶ���ֱ�Ӽ��ȵ�ԭ����________________��ͼ3���жദ���ԵĴ���a����ȴˮ�����������b��___________________��
��4����ƿ���ڱ�ˮ�е�Ŀ����____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȫ��������ԭ�����һ�����Ϳɳ��أ���ͬ��̬�ĺ���������Ϊ���������Ļ������ʣ��ֱ��ڸ��Ե����Ե��Һ�����С���ṹԭ����ͼ��ʾ���õ�طŵ�ʱ���Ҳ��еĵ缫��ӦΪ��V2+-e-=V3+������˵����ȷ����

A. �ŵ�ʱ���Ҳ۷�����ԭ��Ӧ
B. �ŵ�ʱ����۵ĵ缫��Ӧʽ��VO2++2H++e-=VO2++H2O
C. ���ʱ��ÿת��1mol���ӣ�n(H+)�ı仯��Ϊ1mol
D. ���ʱ���������ҺpH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪Ǧ���صĹ���ԭ��Ϊ��Pb��PbO2��2H2SO4![]() 2PbSO4��2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol����ʱ���缫����������11.2 g����ش��������⡣
2PbSO4��2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol����ʱ���缫����������11.2 g����ش��������⡣

��1��A��Ǧ���ص�________����Cu�缫��________�����ŵ�����е��Һ���ܶ�________(������С��������������������)��
��2��Ag�缫�ĵ缫��Ӧʽ��______________________________���õ缫�ĵ缫���ﹲ________g��
��3��Cu�缫�ĵ缫��Ӧʽ��______________________________��CuSO4��Һ��Ũ��________(������С��������������������)
��4����ͼ��ʾ�����й�����ij����(������x)��ʱ��ı仯���ߣ���x��ʾ________��

a����U�ι��в�������������
b����U�ι������������ļ�����
c����U�������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com