
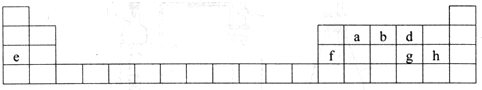
| ���� ���� | IA | ��A | IIIA | ��A | ��A | ��A | ��A | 0�� |
| 1 | �� | |||||||
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� |
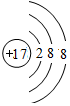 �����Ӱ뾶������S 2-��
�����Ӱ뾶������S 2-��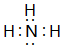 ���ݵ�һ��������ʵ���ɫ���京�еĻ�ѧ�����������Ӽ����ۼ�������ʽΪ
���ݵ�һ��������ʵ���ɫ���京�еĻ�ѧ�����������Ӽ����ۼ�������ʽΪ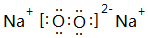 ��
�� ���� ����Ԫ�������ڱ��е�λ��֪���١���ֱ���H��N��O��F��Na��Mg��Al��S��Cl��KԪ�أ�
��1��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С����ԭ�ӵõ������������ӣ����������Ӻ�����18�����ӡ�������������17�����ӵ��Ӳ���Խ�������Ӱ뾶Խ���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С��
��2��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ����O��FԪ�س��⣻Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��
��3���ݺ͢ߵ�����������Ӧˮ����ֱ���NaOH��Al��OH��3�����߷�Ӧ����NaAlO2��ˮ��
��4������������Nԭ�Ӻ�ÿ��Hԭ���γ�1�����õ��Ӷԣ���Nԭ�ӻ���һ���µ��Ӷԣ����������д������Ӽ����ۼ��������Ӻ���������֮��������Ӽ���O-Oԭ��֮����ڹ��ۼ���
��� �⣺����Ԫ�������ڱ��е�λ��֪���١���ֱ���H��N��O��F��Na��Mg��Al��S��Cl��KԪ�أ�
��1��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С����ݡ���Ԫ��λ��ͬһ���ڣ�ԭ�Ӱ뾶��С����Cl����ԭ�ӵõ������������ӣ����������Ӻ�����18�����ӡ�������������17��������ԭ�ӽṹʾ��ͼΪ �����ӵ��Ӳ���Խ�������Ӱ뾶Խ���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С���������Ӱ뾶������S 2-��
�����ӵ��Ӳ���Խ�������Ӱ뾶Խ���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С���������Ӱ뾶������S 2-��
�ʴ�Ϊ��Cl�� ��S 2-��
��S 2-��
��2��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ����O��FԪ�س��⣬�����O��FԪ����ǽ�������ǿ��Ԫ����ClԪ�أ���������ǿ����HClO4��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ����������ǿ����KԪ�أ�������������ˮ���������ǿ����KOH��
�ʴ�Ϊ��HClO4��KOH��
��3���ݺ͢ߵ�����������Ӧˮ����ֱ���NaOH��Al��OH��3�����߷�Ӧ����NaAlO2��ˮ�����ӷ���ʽΪOH-+Al��OH��3=AlO2-+2H2O��
�ʴ�Ϊ��OH-+Al��OH��3=AlO2-+2H2O��
��4������������Nԭ�Ӻ�ÿ��Hԭ���γ�1�����õ��Ӷԣ���Nԭ�ӻ���һ���µ��Ӷԣ�����ʽΪ �����������д������Ӽ����ۼ��������Ӻ���������֮��������Ӽ���O-Oԭ��֮����ڹ��ۼ�������ʽΪ
�����������д������Ӽ����ۼ��������Ӻ���������֮��������Ӽ���O-Oԭ��֮����ڹ��ۼ�������ʽΪ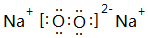 ��
��
�ʴ�Ϊ�� �����Ӽ����ۼ���
�����Ӽ����ۼ���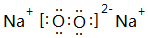 ��
��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã�Ϊ��Ƶ���㣬��ȷԭ�ӽṹ��Ԫ�����ڱ��ṹ��Ԫ���������ǽⱾ��ؼ����漰��ѧ������ӷ���ʽ��д��Ԫ�������ɵ�֪ʶ�㣬ע��Ԫ��������������������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����H��0����S��0���κ��¶��¶����Է����� | |
| B�� | ����H��0����S��0���κ��¶��¶������Է����� | |
| C�� | ��Ҫ���Ȳ��ܹ����еĹ��̿϶������Է����� | |
| D�� | ���Է�������һ�������¿��ܱ���Է����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
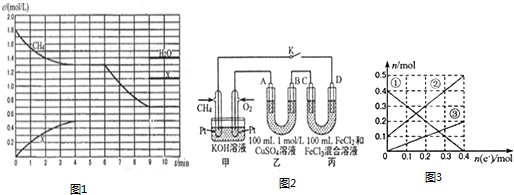
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
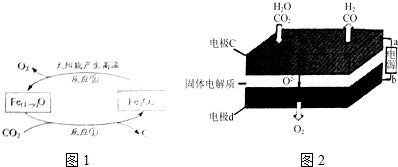
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
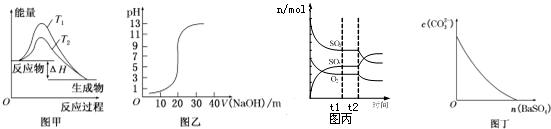
| A�� | ͼ�ױ�ʾ�¶�T1��T2��SO2��O2��Ӧ�����е������仯 | |
| B�� | ͼ�ұ�ʾ0.100 0 mol/L NaOH��Һ�ζ�20.00ml 0.100 0 mol/L CH3COOH��Һ���õ��ĵζ����� | |
| C�� | ͼ����ʾһ�������½��еķ�Ӧ2SO2+O2?2SO3���ɷֵ����ʵ����仯��t2ʱ�̸ı��������������С������� | |
| D�� | ͼ����ʾ�ڱ���Na2CO3��Һ����BaSO4�������Һ��c��CO32-��Ũ�ȱ仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| t�� | 400 | 500 | 800 | 1000 |
| K | 2.6 | 1.6 | 1.0 | 0.40 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʵ������������������ֱ���ȫȼ�գ����߷ų������� | |
| B�� | ��ϡ��Һ�У�H+��aq��+OH-��aq���TH2O��l����H=-57.3 kJ•mol-1��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų�����������57.3 kJ | |
| C�� | ��C��ʯī���TC�����ʯ����H=1.90 kJ•mol-1��֪�����ʯ��ʯī�ȶ� | |
| D�� | ��100 kPaʱ��2 g H2��ȫȼ������Һ̬ˮ���ų�285.8 kJ��������H2ȼ�յ��Ȼ�ѧ����ʽΪ2H2��g��+O2��g���T2H2O��l����H=-285.8 kJ•mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ˮ�еμ�FeCl3��Һ�Ʊ�Fe��OH��3���壺Fe3++3H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3��+3H+ | |
| B�� | ����ʯ��ˮ��ϡ���ᷴӦCa��OH��2+2H+�TCa2++2H2O | |
| C�� | ϡ��������⣺Fe2O3+6H+�T2Fe3++3H2O | |
| D�� | �����Ba��OH��2��Ӧ��Ba2++OH-+SO42-+H+�TBaSO4��+H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com