
��1 200 ��ʱ����Ȼ���������лᷢ�����з�Ӧ
H2S(g)��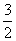 O2(g)===SO2(g)��H2O(g)����H1
O2(g)===SO2(g)��H2O(g)����H1
2H2S(g)��SO2(g)===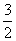 S2(g)��2H2O(g)����H2
S2(g)��2H2O(g)����H2
H2S(g)�� O2(g)===S(g)��H2O(g)����H3
O2(g)===S(g)��H2O(g)����H3
2S(g)===S2(g)����H4
��H4����ȷ����ʽΪ
A����H4�� (��H1����H2��3��H3)
(��H1����H2��3��H3)
B����H4�� (3��H3����H1����H2)
(3��H3����H1����H2)
C����H4��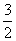 (��H1����H2��3��H3)
(��H1����H2��3��H3)
D����H4��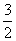 (��H1����H2��3��H3)
(��H1����H2��3��H3)

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��W��Ԫ�����ڱ���ԭ������������������ֶ�����Ԫ�أ��������Ϣ���±���

��1��Wλ��Ԫ�����ڱ��� ���ڵ� �壻W��ԭ�Ӱ뾶��X�� �����С������
��2��Z�ĵ�һ�����ܱ�W�� �����С������  ��̬��Ϊ��̬����˷��������������� ����Ԫ�ء�
��̬��Ϊ��̬����˷��������������� ����Ԫ�ء� ��
��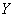 ��ԭ�ӿɹ�ͬ�γɶ��ַ��ӣ�д������һ�����γ�ͬ�ַ��Ӽ�������������� ��
��ԭ�ӿɹ�ͬ�γɶ��ַ��ӣ�д������һ�����γ�ͬ�ַ��Ӽ�������������� ��
��3�����£���Z���������ᷴӦ�����ɫ��Һ�еμ�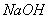 ��Һֱ���������ܹ۲쵽�������ǣ�
��Һֱ���������ܹ۲쵽�������ǣ�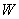 �ĵ���������ᷴӦ����������ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
�ĵ���������ᷴӦ����������ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����25�㡢101 �£���֪13.5g��
�£���֪13.5g��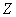 ���嵥����
���嵥���� ��������ȫȼ�պ�ָ���ԭ״̬������419
��������ȫȼ�պ�ָ���ԭ״̬������419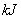 ,�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
,�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
25
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC2�ľ���Ϊ���Ӿ��壬���������������ӵĸ�����Ϊ1��1��D�Ķ��������ӱ�C�ļ������Ӷ�һ�����Ӳ㡣AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�Eԭ������Ϊ26���ش��������⣺(����ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)��
(1)E�����ڱ��е�λ��__________________________________________(ָ�����ں���)��
(2)A��B��C�ĵ�һ��������С�����˳��Ϊ__________________________________��
(3)B���⻯��ķ��ӿռ乹����________��AC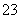 ������ԭ�Ӳ�ȡ________�ӻ���
������ԭ�Ӳ�ȡ________�ӻ���
(4)д������һ��������Ԫ����ɵ���DC2�е������ӻ�Ϊ�ȵ���������Ļ�ѧʽ________________________________________________________________________��
(5)E�ĸ�̬���Ӻ͵ͼ�̬���Ӿ�����A��B��Ԫ�ذ�ԭ�Ӹ�����1��1�γɵĴ�һ����λ����ɵ��������������λ�������ӡ�д���������ӵĻ�ѧʽ________________________(��дһ��)��
(6)X�����ڱ��е縺������Ԫ�أ���Ԫ����DԪ����ɵ����ӻ�����ľ�������ͼ��ʾ�������ӻ�����Ļ�ѧʽΪ________��
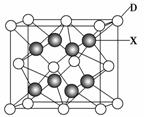
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��2Zn(s)��O2(g)===2ZnO(s)
��H����701.0 kJ��mol��1
2Hg(l)��O2(g)===2HgO(s)����H����181.6 kJ��mol��1
��ӦZn(s)��HgO(s)===ZnO(s)��Hg(l)�Ħ�HΪ
A����519.4 kJ��mol��1���� B����259.7 kJ��mol��1
C����259.7 kJ��mol��1 D����519.4 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��2H2(g)��O2(g)===2H2O(l)
��H����571.6 kJ��mol��1
2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)
��H����1 452 kJ��mol��1
H��(aq)��OH��(aq)===H2O(l)
��H����57.3 kJ��mol��1
����˵����ȷ����
A��H2(g)��ȼ����Ϊ571.6 kJ��mol��1
B. H2SO4(aq)��
H2SO4(aq)�� Ba(OH)2(aq)===
Ba(OH)2(aq)=== BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1
BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1
C��ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų���������
D��3H2(g)��CO2(g)===CH3OH(l)��H2O(l)
��H����135.9 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���ʵ���������ó��Ľ����У�һ����ȷ����(����)
A����ɫ��Һʹ��ɫʯ����ֽ��죬���ۣ���Һ������
B����ɫ��Һ��ɫ��Ӧ�ʻ�ɫ�����ۣ���Һ��������Һ
C����ɫ��Һ����Na2CO3��Һ������ɫ���������ۣ���Һ�к�Ca(OH)2
D����Һ�м�������������Һ�����Ȳ���������ʹʪ��ĺ�ɫʯ����ֽ���������ۣ���Һ�к�NH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���ĽṹΪ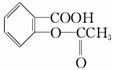 ����һ�������´��л�����ܷ����ķ�Ӧ��
����һ�������´��л�����ܷ����ķ�Ӧ��
(����)
���кͷ�Ӧ����������Ӧ������ȥ��Ӧ����������Ӧ
�ݼӳɷ�Ӧ����ˮ�ⷴӦ
A���ڢ� B���٢ڢ�
C���٢ܢݢ� D���٢ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ܶ�Ϊ1.84g/cm3��Ũ��Ϊ98%��ŨH2SO4�����ʵ�����Ũ��Ϊ( )
A��18.4mol/L B��9.2mol/L C��13mol/L D��12mol/L
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com