
�����������ж���������Σ���ܴ���˺�����ˮ������д��������ŷš�
��֪��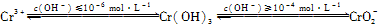
��1���ں���6�۸��ķ�ˮ�м���һ���������������������ʹ��6�۸���ԭ�ɣ�3�۸����ٵ�����ҺpH��6��8֮�䣬ʹFe3����Cr3��ת��ΪFe(OH)3��Cr(OH)3��������ȥ��
��д��Cr2O72-��FeSO4��Һ�����������·�Ӧ�����ӷ���ʽ��________________��
�������ӷ���ʽ��ʾ��ҺpH���ܳ���10��ԭ��____��
��2��������6�۸��ķ�ˮ��������ڣ������������������������Ȼ��ƽ��е�⡣���������ɵ�Fe2����Cr2O72-������Ӧ�����ɵ�Fe3����Cr3������������OH����ϳ����������������ȥ��
��д�������ĵ缫��Ӧʽ��________________��
�ڵ�ⷨ�м����Ȼ��Ƶ�������________________��
��3������ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�С�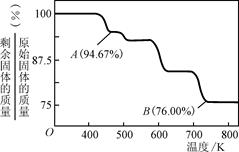
��CrO3����ǿ�����ԣ������л���(��ƾ�)ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬCrO3����ԭ����ɫ�������[Cr2(SO4)3]����÷�Ӧ�Ļ�ѧ����ʽΪ____��
��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯����ͼ��ʾ����B��ʱʣ�����ijɷ���________________(�ѧʽ)��
��1����Cr2O72-��6Fe2����14H��=2Cr3����6Fe3����7H2O
��Cr(OH)3��OH��=CrO2-��2H2O
��2����2H����2e��=H2��(��2H2O��2e��=H2����2OH��)
����ǿ��Һ������
��3����4CrO3��3C2H5OH��6H2SO4=2Cr2(SO4)3��3CH3COOH��9H2O
��Cr2O3
�����������������1�������������л�ԭ�ԣ�Cr2O72-��ǿ�����ԣ������ܷ���������ԭ��Ӧ�����������ӱ����������������ӣ�Cr2O72-����ԭΪCr3+����Ӧ����ʽΪ��Cr2O72-+6Fe2++14H+�T2 Cr3++6Fe3++7H2O���ʴ�Ϊ��Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O�����������֪����pH����10��c��OH-����10-4mol?L-1ʱ��Cr��OH��3ת���CrO2-��Cr��OH��3+OH-�TCrO2-+2H2O���ʴ�Ϊ��Cr��OH��3+OH-�TCrO2-+2H2O����2���ٽ���+6�۸��ķ�ˮ��������ڣ������������������������Ȼ��ƽ��е�⣬��������ʧ�������ɶ��������ӣ������������ӵõ��������������缫��ӦʽΪ��2H++2e-�TH2����2H2O+2e-�TH2��+2OH-���ʴ�Ϊ��2H++2e-�TH2����2H2O+2e-�TH2��+2OH-����ˮ��������ʣ���������ˮ�ĵ���������С���Ȼ�����ǿ����ʣ���ˮ������ȫ���뵼����Һ��������Ũ��������������ǿ��Һ�����ԣ��ʴ�Ϊ����ǿ��Һ�����ԣ���3����CrO3����ǿ�����ԣ������л����ƾ���ʱ���Ҵ������������ᣬ̼��ƽ�����ϼ۴�-2�����ߵ�0��1���Ҵ����ϼ۱仯4��CrO3����ԭ����ɫ�������[Cr2��SO4��3]�����Ļ��ϼ۴�+6�۽��͵�+3�ۣ�1��CrO3���ϼ۱仯3�����ߵ���С��������12���ٸ���ԭ���غ�ã�4CrO3+3C2H5OH+6H2SO4=2Cr2��SO4��3+3CH3CO0H+9H2O���ʴ�Ϊ��4CrO3+3C2H5OH+6H2SO4=2Cr2��SO4��3+3CH3CO0H+9H2O������CrO3������Ϊ100g����CrO3�и�Ԫ�ص�����Ϊ��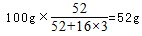 ����B��ʱ���������Ϊ��100g��76%=76g��Co������û�б䣬������������Co������Ϊ52g����Ԫ�ص�����Ϊ16�����ߵĸ�����Ϊ
����B��ʱ���������Ϊ��100g��76%=76g��Co������û�б䣬������������Co������Ϊ52g����Ԫ�ص�����Ϊ16�����ߵĸ�����Ϊ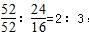 ������B��ʱʣ�����ijɷ���Cr2O3���ʴ�Ϊ��Cr2O3��
������B��ʱʣ�����ijɷ���Cr2O3���ʴ�Ϊ��Cr2O3��
���㣺���⿼�������ӷ���ʽ�͵缫��Ӧʽ����д���ѶȲ���ע�⣨3�������У��ڱ仯�����У�Co������û�б䣬�ǽ���Ĺؼ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������A��B����ѧ���������ʣ����������ӿɴ��±���ѡ��
| ������ | K����Na����Fe2����Ba2����NH4+ |
| ������ | OH����NO3����I����HCO3����AlO2����HSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������̫�����屬�������������ˮ�ʵ����ض���Щ�¼��ٴ��������ҹ�ˮΣ���ľ��ӡ���̫������ij�������ŷŵ���ˮ�У�������ijЩ�������к������ʣ�������Ϊ���ܺ���Fe3����Ba2����K����OH����Cl���� ��
��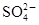 ��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��ȡ��ˮ��ϸ�۲죬����ɫ��������һ״̬��
����ȡ������ˮ�У�����ϡ���ᣬ�д�����ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ��
����pH��ֽ�ⶨ��ˮ��pH����ֽ������ɫ��
(1)�ɴ˿�֪������ˮ�п϶����е�������___________���϶�û�е�������___________�����ܺ��е�������___________��
(2)���ͨ��ʵ���һ��ȷ����Һ�п��ܴ��ڵ�����__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����з�Ӧ�����ӷ�Ӧ����ʽ��ȫ�Ե�10�֣��д���Ϊ0�֣�
��1��NaAlO2��Һ�м�������������
��2��AlCl3��Һ�м��������NaOH
��3��Al2O3��NaOHˮ��Һ�ķ�Ӧ
��4��Fe3O4�ܽ���ϡ������
��5��FeCl2��Һ�м����ữ��H2O2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ�е������������еļ������ӹ��ɣ�Na+��Fe3+��Cu2+��Ba2+��AlO2����CO32����SO32����SO42����ȡ����Һ�����й�ʵ�飬ʵ�鲽�輰������£�
������Һ�м������ϡ���ᣬ�õ��������Һ�ף�
������Һ���м������NH4HCO3��Һ���õ���ɫ�����ҡ������Һ���Һ�ң�
������Һ���м������Ba(OH) 2��Һ���õ���ɫ�����������������Һ����
�ܼ�������ס������ҡ����������ֻ����һ�ֳɷݣ����Ҹ�����ͬ��
��ش��������⣺
��1��ֻ����ʵ����ܵó��Ľ������� ��3�֣�
��2����������һ������ ��1�֣������ܺ��� ��1�֣�
��3������Һ�п϶����ڵ�������
��4�������ҷ��ӵĽṹʽΪ�� ��
��5������Һ���м���NH4HCO3��Һ���������ù����п��ܷ�����Ӧ�����ӷ���ʽΪ �� �� ��������Ҫ���ɲ����꣬Ҳ�ɲ��䣩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ʻش����⣺�� �������ƹ��塡�� ͭ˿���� �Ȼ������塡�� ϡ������Һ���� ������̼���塡�� ���Ǿ��塡�� �����Ȼ���
���ڵ���ʵ��ǣ� �����ţ���ͬ�������ڷǵ���ʵ��� ���ܵ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I��NaOH��FeCl3����ѧ��ѧʵ���ҳ��õ��Լ���
��1����һ������������NaOH��Һ��Ӧ�Ĺ��嵥����________��________������������
����һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�����ʵ����ƫ�͵�ԭ����________��
| A������ƿ��ԭ����������ˮ |
| B��ϴ���ձ��Ͳ���������Һδת������ƿ�У� |
| C������ʱ�۲�Һ�温�� |
| D���ܽ��δ����ȴ����ת��������ƿ�� |
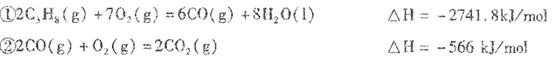
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һƿ�������Һ�����п��ܺ�H+��NH4+��K+��Cu2+��Fe3+��CO32-��I-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飺
����pH��ֽ���飬������Һ��ǿ����
��ȡ������Һ�������������Ƶ���ˮ��������CCl4����CCl4������ɫ
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о���������
�ܽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵȷ�����ش�
��1������Һ�У��϶����ڵ��� ���϶������ڵ������� ��
��2������ȷ���Ƿ���ڵ�������_______��֤����(��)�Ƿ���ڵ�ʵ�鷽���� ��
��3��д��������漰�����ӷ���ʽ�����õ����ű������ת�Ƶķ������Ŀ ��
��4��д��������漰�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ij������Һ�У���Cl-֮����ܻ����е����ʵ����������������е�һ�ֻ���֣�SO32-��CO32-��SiO32-��I-��NO3-��SO42-���ڴ���Һ�м���������ᣬ�������ݣ���Һ��ɫ������Գ��壬����ԭ��Һ�е����������������3�֡��Իش��������⡣
��1��ԭ��Һ���Ƿ���SiO32-�� ����С���û�С������ж������� ���������ӷ���ʽ��ʾ��
��2�����ɵ�������һ���� �������е������� (����ĸ���)��
| A����ɫ��ζ |
| B����ɫ�д̼�����ζ |
| C�����ڴ�����Ⱦ�� |
| D��������ˮ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com