
��һƿ�������Һ�����п��ܺ�H+��NH4+��K+��Cu2+��Fe3+��CO32-��I-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飺
����pH��ֽ���飬������Һ��ǿ����
��ȡ������Һ�������������Ƶ���ˮ��������CCl4����CCl4������ɫ
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о���������
�ܽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵȷ�����ش�
��1������Һ�У��϶����ڵ��� ���϶������ڵ������� ��
��2������ȷ���Ƿ���ڵ�������_______��֤����(��)�Ƿ���ڵ�ʵ�鷽���� ��
��3��д��������漰�����ӷ���ʽ�����õ����ű������ת�Ƶķ������Ŀ ��
��4��д��������漰�����ӷ���ʽ ��
��10�֣�
��1��H+��I-��NH4+�� Cu2+��Fe3+��CO32-
��2��K+����ɫ��Ӧ
��3��Cl2+ 2I- = I2 + 2Cl-
��4��NH4+ + OH-  NH3��+H2O
NH3��+H2O
���������������1����pH��ֽ���飬������Һ��ǿ���ԣ���һ������H+��һ��������CO32-�������������Ƶ���ˮ��������CCl4����CCl4������ɫ��һ������I-����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о��������ɣ���һ��������Cu2+��Fe3+�����õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ��������һ������NH4+��
��2����ɫ��Ӧ��֤���Ƿ���K+��
��3��������ǿ������������������ǿ�Ļ�ԭ����������Ӧ�����ɵⵥ�ʣ�
��4��������ڼ��ȵ��������ǿ�ᷴӦ���ɰ�����
���㣺��������ʶ��


 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
��ѧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������:��ͭ�����Ǣ�CuO��NaHSO4 ��Ba��OH��2�������������ɱ��ᵨ����ˮ
��1���������ʷ����в����ڵ���ʵ��� ������ţ�������ˮ��Һ�еĵ��뷽��ʽΪ ��
��2��̼��������������մ����������ʳ�����ơ���һ�������£���������������������̼������ʳ�ε������� ������ţ���
��3������������������������ˮ��Һ�з�����Ӧ,�����ӷ���ʽΪ:H++OH-=H2O,��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����� ��Һ�л����μӢ� ��Һ�������Һ�պó�����ʱ�����ӷ���ʽΪ��_______��
��5������������������������һ������������ÿ�����һ�������ʡ���ʽ̼��ͭ��Cu2��OH��2 CO3]����д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ж���������Σ���ܴ���˺�����ˮ������д��������ŷš�
��֪��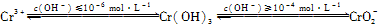
��1���ں���6�۸��ķ�ˮ�м���һ���������������������ʹ��6�۸���ԭ�ɣ�3�۸����ٵ�����ҺpH��6��8֮�䣬ʹFe3����Cr3��ת��ΪFe(OH)3��Cr(OH)3��������ȥ��
��д��Cr2O72-��FeSO4��Һ�����������·�Ӧ�����ӷ���ʽ��________________��
�������ӷ���ʽ��ʾ��ҺpH���ܳ���10��ԭ��____��
��2��������6�۸��ķ�ˮ��������ڣ������������������������Ȼ��ƽ��е�⡣���������ɵ�Fe2����Cr2O72-������Ӧ�����ɵ�Fe3����Cr3������������OH����ϳ����������������ȥ��
��д�������ĵ缫��Ӧʽ��________________��
�ڵ�ⷨ�м����Ȼ��Ƶ�������________________��
��3������ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�С�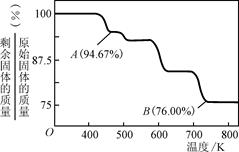
��CrO3����ǿ�����ԣ������л���(��ƾ�)ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬCrO3����ԭ����ɫ�������[Cr2(SO4)3]����÷�Ӧ�Ļ�ѧ����ʽΪ____��
��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯����ͼ��ʾ����B��ʱʣ�����ijɷ���________________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijǿ������ҺX���ܺ���Ba2+��A13+��NH4+��Fe2+��Fe3+��CO32-��SO32-��SO42-��C1-��NO3-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飬ʵ��������£�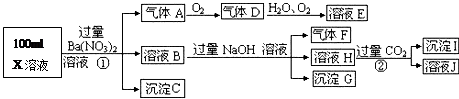
����������Ϣ���ش��������⣺
��1������FΪ
��2�����������У���ҺX�г�H+��϶����е������� ������ȷ���Ƿ��е������� ��
��3��д������A�����ӷ���ʽ��
��4��ͨ����������KClO�ڼ�������������G���Ʊ�һ�����͡���Ч�����ˮ������K2FeO4����д���Ʊ������е����ӷ���ʽ ��
��5������ⶨA��F��I��Ϊ0.1mol��100mL X��Һ��n��H+��=0.4mol���Ҳ���ȷ��������ֻ��һ�֡���X��Һ�в���ȷ���������� �������C�����ʵ���Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij���������Թ�ҵ��ˮ�к���һ������Fe3+��Cu2+��Au3+�����ӡ����������ͼ�еĹ������̣����ó��õ��ᡢ���ҵ�����еķ���м���ӷ�ˮ�л��ս𣬲�����һ���������죨Fe2O3��������ͭ������֪ͭ���ܽ���ϡ��������������ͭ��Һ��
����д����հף�
��1��ͼ�б�Ŵ���������Ӧ���ʷֱ��ǣ��ѧʽ���� ��
�� �� ��
��2��A������ɷ�Ϊ ���ѧʽ��
��3��д���ݴ���Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����K2Cr2O7+14HCl=2KCl+2CrCl3+3Cl2��+7H2O��Ӧ�У�_________���������� _______�ǻ�ԭ����HCl���ֵ�������_______��________��1mol K2Cr2O7��Ӧת�Ƶ��ӵ����ʵ�����_________��
��2�����������Т�ʳ��ˮ��NaOH ��Cu ��HCl����ݿ��� �ƾ� ��CO2��KNO3���ڵ���ʵ��� ������ţ���ͬ�������ڷǵ���ʵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�ӵ������мס��������������������ŷŵij��幤ҵ��ˮ�У�������Na+��Ag+��Fe3+��C1-��OH-��NO3-�������ӡ�
��1�����ⶨ�׳��ķ�ˮ���Գ����ԣ����ҳ���ˮ����������������Ӧ���� ��
��2�����Ҫ���շ�ˮ�еĽ������������ڷ�ˮ�м��������һ�ֳ�����������Ӧ�����Һ��ֻ��һ�ֽ��������ӣ��������ù���ijɷ��� ��Ҫ��һ����������������� ��
��3�����׳����ҳ��ķ�ˮ���ʵ��ı�����ϣ�����ʹ��ˮ�е�ijЩ����ת��Ϊ������д�����ɺ��ɫ���������ӷ���ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ������������
(1)д���������ʵĵ��뷽��ʽ:
Fe2(SO4)3_____________________________________________��
NaHCO3______________________________________________��
(2)д�����з�Ӧ�����ӷ���ʽ��
ϡ������̼��Ʒ�Ӧ___________________________________��
����������Һ��ϡ���ᷴӦ_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ��Һ�������п��ܴ����������ӣ�Na+��Ag+��Ba2+��Al3+��AlO2-��S2-��CO32-��SO32-��SO42-����ȡ����Һ�����й����飬�������£�
�ش��������⣺
��1�����ɳ��������ӷ���ʽ�� ��
��֪�������������������ɣ�������ˮ������HBr�����������ʵ����һ���������ֳɷ֣��������Լ��Լ����������±��У��ɲ�����������ÿһ�ж�Ӧ��ȷ���ɵ÷֣�
| ���� | ʵ��Ŀ�� | �Լ� | ���� |
| 1 | | | |
| 2 | | | |
| 3 | | | |
| 4 | | | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com