
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ��
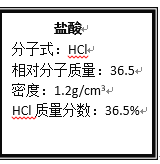
�Ը����й����ݻش��������⣺
(1)��Ũ��������ʵ���Ũ��Ϊ___________mol/L��
(2)ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����__________��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl������Ŀ D����Һ���ܶ�
(3)ijʵ����Ҫ480 mL���ʵ���Ũ��Ϊ0.3 mol/Lϡ���ᡣijѧ��������Ũ���������ˮ�������ơ�
�ٸ�ѧ����Ҫ����Ͳ��ȡ___________ mL����Ũ����������ơ���ʵ���в�������������_________________________________��
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�_________________��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ��Լ30mL�����ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��500mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿���� ��ƫ�ߡ���ƫ�͡�����Ӱ�족����
����Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��_________________��
��Һע������ƿǰû�лָ������¾ͽ��ж���_________________��
����ƿ��ˮϴ�����ϡ������ϴ����û�и����ֱ��ʹ��_________________��
���𰸡�12 BD 12.5 ���衢���� BCAFED ƫ�� ƫ�� ƫ��
��������
��1������c=![]() �������Ũ��������ʵ���Ũ�ȣ�
�������Ũ��������ʵ���Ũ�ȣ�
��2���κ���Һ���Ǿ�һ�ȶ��ķ�ɢϵ���κ�������ܶȺ�Ũ�Ȳ��䣻
��3����A������450mL��Һ����Ҫ����500mL��Һ������500mL 0.3mol/Lϡ�������Ȼ�������ʵ����������ҪŨ����������
�ڸ�������һ�����ʵ���Ũ�ȵ���Һ���Ʋ����������
�۸���c=![]() �ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
(1)����������ΪVL�������ʵ�����ΪV��1000mL��1.2gcm-3��36.5%�����ʵ����ʵ���Ϊ![]() =12Vmol���������ʵ����ʵ���Ũ��Ϊ
=12Vmol���������ʵ����ʵ���Ũ��Ϊ![]() =12mol/L��
=12mol/L��
(2) �κ���Һ���Ǿ�һ�ȶ��ķ�ɢϵ���κ�������ܶȺ�Ũ�Ȳ��䣬�������ͬʱ�����ʵ����ʵ�����ͬ����Һ�����ӵ���ĿҲ��ͬ��
��ѡBD��
(3) ������480mL���ʵ���Ũ��Ϊ0.3mol/Lϡ���ᣬʵ����û��480mL����ƿ��������Ҫѡ��500mL����ƿ�����Ƶ���Һ���Ϊ500mL������500mL 0.3mol/L��ϡ���ᣬ��Ҫ12.0mol/L�����Ϊ��![]() =0.0125L=12.5mL��
=0.0125L=12.5mL��
������500mL 0.3mol/L��ϡ���ᣬ��������Ϊ�����㡢��ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ��������Բ���˳��Ϊ��BCAFED��
������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬������ȡ��Ũ�������ƫС������c=![]() �ɵã����Ƶ���ҺŨ��ƫ�ͣ�
�ɵã����Ƶ���ҺŨ��ƫ�ͣ�
��Һע������ƿǰû�лָ������¾ͽ��ж��ݣ��ȵ���Һ���ƫ����ȴ�������С���������Ƶ���Һ���ƫС����ҺŨ��ƫ�ߣ�
����ƿ��ˮϴ�����ϡ������ϴ����û�и����ֱ��ʹ�ã��������������ƫ�࣬�����ҺŨ��ƫ�ߡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʱ仯�ͷ����˵����ȷ���ǣ� ��
A. ��������ˮ�������������������ǻ����
B. �������̬��Al2O3��12Cת��Ϊ14C�����ڻ�ѧ�仯
C. ��������Һ�͵�����Һ�ı����������ܷ���������ЧӦ
D. SiO2��NO2��Al2O3����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������NOCl�����ںϳ������ȡ�������Cl2��NO�ڳ��³�ѹ�ºϳɣ������۵�Ϊ-64.5�棬�е�Ϊ-5.5�棬�������ǻ�ɫ���ж����壬��ˮ��ˮ�⡣ �밴Ҫ��ش�����������⣺
(1)������м��ϡ�����ַ�Ӧ�Ʊ�NO�����ӷ���ʽΪ��______________________��
(2)�Ʊ�NOCl��װ������ͼ��ʾ������˳��Ϊ:a��_________________________(�������������ҷ�����Сд��ĸ��ʾ)��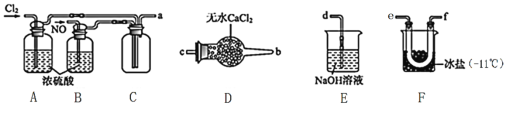
��װ��A��B�����Ǣٸ���NO��Cl2����___________________________________________��
��װ��D��������______________________________________��
��װ��E��NOCl������Ӧ�Ļ�ѧ����ʽΪ________________��
(3)��ҵ����������NOβ�����������ж��֣����м�ӵ绯ѧ������ԭ����ͼ��ʾ��
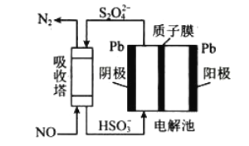
�ù����������ĵ缫��ӦʽΪ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ--ѡ��5���л���ѧ����]
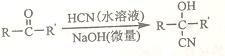
�����ϩ�����ڼ����������ܿ��پۺ�Ϊ ���Ӷ����н���ԣ�ij�������ϩ������G���ĺϳ�·�����£�
���Ӷ����н���ԣ�ij�������ϩ������G���ĺϳ�·�����£�
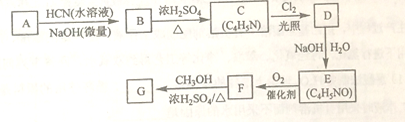
��֪��
��A����Է�����Ϊ58����Ԫ����������Ϊ0.276���˴Ź���������ʾΪ����
��
�ش��������⣺
��1��A�Ļ�ѧ����Ϊ_______��
��2��B�Ľṹ��ʽΪ______����˴Ź���������ʾΪ______��壬�������Ϊ______��
��3����C����D�ķ�Ӧ����Ϊ________��
��4����D����E�Ļ�ѧ����ʽΪ___________��
��5��G�еĹ�������___�� ____ ��_____��������������ƣ�
��6��G��ͬ���칹���У���G������ͬ���������ܷ���������Ӧ�Ĺ���_____�֡������������칹��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ� ��
A. 2.4gMg�ڿ�����ȼ������MgO��Mg3N2ת�Ƶĵ�����Ϊ0.2NA
B. ��״���£�18gH2O�������22.4L
C. 0.1L3mol��L-1NH4NO3��Һ�к��е�NH4+����ĿΪ0.3NA
D. 1molCl2ͨ������ʯ��ˮ��ת�Ƶĵ�����Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��![]() ��Cl-��Mg2+��Ba2+��
��Cl-��Mg2+��Ba2+��![]() ��
��![]() ����ȡ����100mL��Һ��������ʵ�飺
����ȡ����100mL��Һ��������ʵ�飺
��һ�ݼ���AgNO3��Һ�г���������
�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ���0.08mol���壻
�����ݼ�����BaCl2��Һ�ø������12.54g������������ϴ�ӡ������������Ϊ4.66g��
����֪�� ![]() +OH-
+OH-![]() H2O+NH3����
H2O+NH3����
��������ʵ�飬�ش��������⡣
��1���ɵ�һ�ݽ��е�ʵ���ƶϸû�����Ƿ�һ������Cl-��____________�����ǻ��
��2���ɵڶ��ݽ��е�ʵ���֪�������Ӧ����____________�������ӷ��ţ��������ʵ���Ũ��Ϊ____________��
��3���ɵ����ݽ��е�ʵ���֪12.54g�����ijɷ�Ϊ____________��
��4���ۺ�����ʵ�飬����Ϊ���½�����ȷ������______��
A���û��Һ��һ������K+��![]() ��
��![]() ��
��![]() �����ܺ�Cl-����n��K+����0.04mol
�����ܺ�Cl-����n��K+����0.04mol
B���û��Һ��һ������![]() ��
��![]() ��
��![]() �����ܺ�K+��Cl-
�����ܺ�K+��Cl-
C���û��Һ��һ������![]() ��
��![]() ��
��![]() �����ܺ�Mg2+��K+��Cl-
�����ܺ�Mg2+��K+��Cl-
D���û��Һ��һ������![]() ��
��![]() �����ܺ�Mg2+��K+��Cl-
�����ܺ�Mg2+��K+��Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���һ�ֽϳ�ʹ�õĻ��ʣ����ڳ������ֽ⡣ij��ѧ��ȤС���̼淋ijɷִ������ʣ�����������̽����
������Һ�е����������ӣ�ȡ������������Թ��У��������ᣬ�����ɵ�����ͨ�����ʯ��ˮ�У��а�ɫ�������ɡ�����ȡ����̼立����Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�������ɵ����壬ʯ����ֽ����ɫ��
��1������ʵ�������Ʋ�̼��������������ӿ�����____��_____��
��2������ʵ������̼�������NaOH��Һ���ȷ�Ӧ�����ӷ���ʽ������_____��
�ⶨ̼���CԪ�غ�NԪ�������ȡ�����ȤС��ȷ��ȡag̼泥�����ʹ֮�ֽ⣬���Ѳ���ͨ���ʯ���У���ͼ1��ʾ��

��3��̼粒���Ӧ����____�н��м��ȡ�
A���Թ� B�������� C����ƿ D������
��4���Ӱ�ȫ�ĽǶȿ��ǣ�β��������װ�ÿ���ѡ����ͼ2_____��
��5�������պ�û�й�����࣬����U�ι���ʵ��ǰ���������Ϊbg���ɴ˲��NԪ�ص�������____g��
��6��Ϊ�˲ⶨ̼���̼Ԫ�ص�������������Ƶ�ʵ�鷽���ǽ�ag̼���ȫ�ܽ���ˮ���������BaCl2��Ȼ��ⶨ���ɳ����������������۸÷����Ƿ������_____�����������������������������_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Journal of Energy Chemistry����־�������ҹ���ѧ����Ƶ�CO2���β�����ת��װ�ã���ʾ��ͼ��ͼ��ʾ�������й�˵����ȷ����
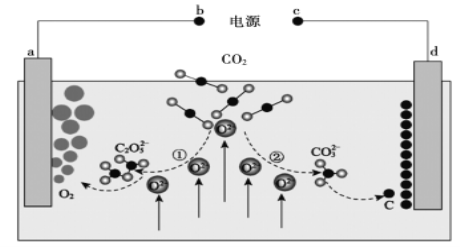
A. b����
B. �٢��У�����CO2ʱ̼Ԫ�صĻ��ϼ۷����˱仯
C. a���缫��ӦʽΪ![]()
D. ת��1mol���ӿɲ���CO2����22.4L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽���������Ļ������һЩ��ѧ���ʣ�ijѧ��ʵ��С�����������ʵ�顣

��1����A��B��Һ�зֱ���뼸��KSCN��Һ�� A��Һ��ɫ��________B��Һ��ɫ_______��
��2��д�������������йط�Ӧ�����ӷ���ʽ��A��B___________________������2mol��A����÷�Ӧ����ת��______mol���ӡ�A��_____��
��3��B��C����¶�ڿ����У��ɿ�����������__________________________________��
��4����A�ı�����Һ�Ƴɽ���IJ���______________________��������ͨ���ý���ʱ���ɿ���һ�������ġ�ͨ·�������������Ϊ___________ЧӦ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com