
ijУͬѧΪ̽��Br2��I2��Fe3����������ǿ��������������ʵ�顣
ʵ��٣�ȡ����KI��Һ���Թ��У��ȼ�����ˮ�����ټ���CCl4�����ã��۲쵽�²�Һ����Ϻ�ɫ��
ʵ��ڣ�ȡ����FeSO4��Һ���Թ��У��ȼ�����ˮ�����ټ����μ�����KSCN��Һ�����۲쵽��Һ�ʺ�ɫ��
��1��д�����ӷ���ʽ��
ʵ��٣�______________________________________________________��
ʵ��ڣ�______________________________________________________��
��2������������ʵ�飬�����ʵ������Կ��Եó�����ȷ������________��
| A��Br2>I2 | B��Fe3��>Br2 | C��Br2>Fe3�� | D��I��>Br�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺�����������ʵ��ش����⣺
(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ�����������________��
(2)����������Ҫ�Ĺ�ҵ���ϣ��÷���м�Ʊ���������������ʾ��
�ش��������⣺
�ٲ��������õķ���������������________���������������________���ò����ľ��巽����________��
��Na2CO3��Һ���Գ����ۣ�ԭ����(�����ӷ���ʽ��ʾ)________��
����д������FeCO3���������ӷ���ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����NaOH��Һ����FeSO4��Һ�У��ܹ۲쵽�������� ��
��2����һ�������۷����Ȼ�����Һ�У���ȫ��Ӧ��������Һ��Fe2+��Fe3+��Ũ��ǡ����ȡ�
��Fe�ۺ��Ȼ��������ʵ���֮��Ϊ________
��3����40mL 1mol/L��NaOH��Һ��ͨ�������CO2��������Һ�ֳ����ȷݡ�
��һ�ݼ��ȡ����ɡ����գ����ù���Ļ�ѧʽ�� ��
�ڽ�NaHSO4���������һ����Һ�У�������Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƣ�NaClO2��������ˮ��������ɰ�ǡ���֬��Ư����ɱ�����������ù������ⷨ�����������ƵĹ�������ͼ��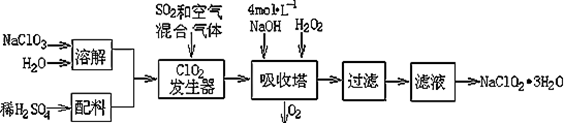
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O ;
��Ksp(FeS)��6��3��10-18 �� Ksp(CuS)��6��3��10-36 ��Ksp(PbS)��2��4��10-28
��1���������ڷ�����Ӧ�����ӷ���ʽΪ ���ù��������е�NaClO3��ClO2��NaClO2����ǿ�����������Ƕ��ܺ�Ũ���ᷴӦ��ȡCl2�����ö������Ⱥ�Ũ������ȡCl2��������5 mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ�����������Ϊ g��
��2������Һ�еõ�NaClO2��3H2O������������������ ����д��ţ���
a����b���գ�c���ˣ�d��ȴ�ᾧ��e����
��3��ӡȾ��ҵ�����������ƣ�NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2��
�±��� 25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
| ���� | HClO2 | HF | HCN | H2S |
| Ka/mol?L-1 | 1��10-2 | 6��3��10-4 | 4��9��10-10 | K1��9��1��10?8 K2��1��1��10?12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʵ���Һ�ũҵ�������й㷺����;��ʵ�����Զ�������Ϊ��Ҫԭ���Ʊ�������ء��䲿���������£�
��1���ڢٲ���������Ӧ�� �н��У������ô�������ԭ���� ���û�ѧ��ʽ��ʾ����
��2���ڢܲ�ͨ��CO2������ʹMnƬ������Ӧ������MnO4����MnO2����Ӧ�����ӷ���ʽΪ ������ɷ�Ӧʱ��ת��ΪKMnO4��ռȫ��K2MnO4�İٷ���ԼΪ ����ȷ��0��1%����
��3���ڢݲ����ȹ��˵��� ��
��4���ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ�� ��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���ԭ���� ���û�ѧ����ʽ��ʾ����
��5��H2O2��KMnO4�����dz��õ�ǿ������������H2O2��Һ�еμ����Ը��������Һ�������Ը��������Һ����ɫ��д���÷�Ӧ�����ӷ���ʽ�� ���÷�Ӧ˵��H2O2�������Ա�KMnO4 ���ǿ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�������������ɷ�����H1N1���С�����������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��H2SO4��������Na2SO3��Ӧ�Ƶá���д����Ӧ�����ӷ���ʽ_____________________________________________________________________________��
��2��ij��ɫ��Һֻ��������8�������е�ij���֣�Na+��H+��Mg2+��Ag+��Cl-��OH-��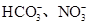 ����֪����Һ����Al2O3��Ӧ����
����֪����Һ����Al2O3��Ӧ����
�ٸ���Һ��Al2O3��Ӧ����Al3+���ɣ���ԭ��Һ��һ������_______��һ�����Ậ�д�����_______��
�ڸ���Һ��Al2O3��Ӧ����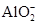 ���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��
���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��
��д������Һ��Al2O3��Ӧ����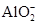 �����ӷ���ʽ____________________________��
�����ӷ���ʽ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��FeCl3��Һ��NH4SCN��Һ��Ϸ������·�Ӧ
��Fe3++SCN- Fe(SCN)2+ K1="200" ��Fe(SCN)2++SCN-
Fe(SCN)2+ K1="200" ��Fe(SCN)2++SCN- Fe(SCN)2+ K2
Fe(SCN)2+ K2
��ɫ ���ɫ
��1����֪������ʼc(Fe3+)��c(SCN-)��Ϊ0.001 mol/L����û����Һ��c(Fe3+)ԼΪ8.5��10-4 mol/L��c[Fe(SCN)2+]ԼΪ1.5��10-4 mol/L��c[Fe(SCN)2+]ԼΪ5��10-6 mol/L����Ӧ�ڵ�ƽ�ⳣ��K2Ϊ ��
��2��ȡ���ݻ����Һ���ֱ�����Һ�еμ�ͬ���ͬŨ�ȵ�FeCl3��Һ��NH4SCN��Һ����Һ��ɫ�������ԭ���� ����ϸ�۲죬�μ�NH4SCN��Һʱ�����Һ��ɫ����һЩ����ԭ���� ��
��3����FeCl2��NH4SCN�����Һ�еμ��ữ��H2O2��Һ����Һ�ȱ�죬д��H2O2��Fe2+��Ӧ�����ӷ���ʽ �������μ�H2O2����Һ��ɫ�����������ܹ�ʹ����ʯ��ˮ����ǵ�������������е�Ԫ��ȫ��ת��ΪN2����Ӧ�����Һ�еμ�BaCl2��Һ���в�����ϡ�����ɫ�������ɡ�д��H2O2��SCN-��Ӧ�����ӷ���ʽ ��
��4��Ϊ�ⶨij��Һ��c(Cl-)��ȡ����ҺV1 mL�������м���V2 mLŨ��Ϊc2 mol/L��AgNO3��Һ�����������ټ���������立�[NH4Fe(SO4)2��12H2O]��ָʾ������NH4SCN����Һ��Ũ��Ϊc3 mol/L���ζ�ʣ���Ag+������NH4SCN��Һ���ΪV3 mL������֪Ag++SCN- = AgSCN��(��ɫ����)��Ksp(AgSCN)=4.9��10-13��Ksp(Ag Cl)=1.56��10-10������ش�
������NH4SCN����Һ�ζ�ǰ��Ӧ��ͨ�����˳�ȥ���ɵ�AgCl���������ˣ���ⶨ����� �����ƫ�ߡ�����ƫ�͡��䣩��
�����ղ��c(Cl-)= mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Χ�������������ʣ���������A12O3����ϡ���ᣬ��H2SO4����Ba(OH)2���壬�ް�ˮ����A12(SO4)3������Ҫ��ش��������⡣
��1��������ǿ�ᷴӦ��������ǿ�Ӧ���� ������ţ���
��2�����ڵ���ʵ��� ������ţ���
��3��д����������ȡAl(OH)3�����ӷ���ʽ ��
��4�������ʷ�����Ӧ�����ӷ���ʽΪH++OH��=H2O����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5��34.2g������ˮ���500mL��Һ����Һ��SO42�������ʵ���Ũ��Ϊ ��
��6������۷�����Ӧ�Ļ�ѧ����ʽΪA1+4HNO3=A1(NO3)3+NO��+2H2O���÷�Ӧ�л�ԭ���������������ʵ���֮���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Na��Ũ��Ϊ0��5mol��L��ij������Һ�У������ܺ����±��е����������ӣ�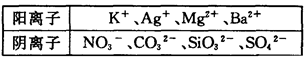
ȡ����Һ100mL��������ʵ�飨��������ڱ�״���²ⶨ����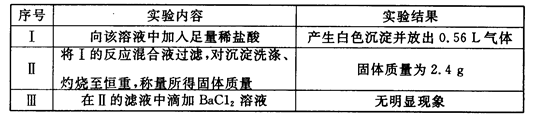
�Իش��������⣺
��1��ʵ�����ȷ��һ�������ڵ���������____________��
��2��ͨ��ʵ���ͱ�Ҫ���㣬��д�±��������ӵ�Ũ�ȣ��ܼ�����ģ���д��������һ�������ڵ������0��������ȷ���Ƿ���ڵ������?����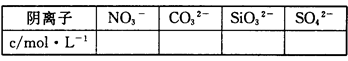
��3���ж�K���Ƿ���ڣ�������������СŨ�ȣ���������˵������_____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com