
��1����NaOH��Һ����FeSO4��Һ�У��ܹ۲쵽�������� ��
��2����һ�������۷����Ȼ�����Һ�У���ȫ��Ӧ��������Һ��Fe2+��Fe3+��Ũ��ǡ����ȡ�
��Fe�ۺ��Ȼ��������ʵ���֮��Ϊ________
��3����40mL 1mol/L��NaOH��Һ��ͨ�������CO2��������Һ�ֳ����ȷݡ�
��һ�ݼ��ȡ����ɡ����գ����ù���Ļ�ѧʽ�� ��
�ڽ�NaHSO4���������һ����Һ�У�������Ӧ�����ӷ���ʽ�� ��
��1���Ȳ�����ɫ������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��
��2��1:5
��3����Na2CO3 ��HCO3����H+=H2O��CO2��
���������������1����NaOH��Һ����FeSO4��Һ�У��ܹ۲쵽�������ǣ��Ȳ�����ɫ������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��
��2����Fe+ 2Fe3+="3" Fe2+������ȫ��Ӧ��Fe2+��Fe3+��Ũ��ǡ����ȣ�������1Fe��2 Fe3+��������3Fe2+����Һ����3 Fe3+����ԭ��Һ����5 Fe3+������Fe�ۺ��Ȼ��������ʵ���֮����1:5
��3�� ����40mL 1mol/L��NaOH��Һ��ͨ�������CO2������̼�����ƣ����ȡ����ɡ����գ�̼�����Ʒֽ⣬���յ�Na2CO3����
��NaHCO3��NaHSO4��Ӧ�����ӷ���ʽ����ע��������ʽ�εĵ�����ʽ��NaHCO3= HCO3����Na+��NaHSO4= Na++ H++ SO42-���������ӷ���ʽ��HCO3����H+=H2O��CO2��
���㣺����NaOH��Һ��FeSO4��Һ��Ӧ��ʵ��������Ԫ�صļ��㡢̼�����ƵIJ��ȶ��ԡ���ʽ�εĵ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�ְ�ɫ��ĩ���������������Ӻ��������еļ��֡�
�����ӣ�S2����Cl����NO�� ��
�� ��
�� ��
�� ��
��
�����ӣ�Na����Mg2����Al3����Ba2����Fe2����Fe3����Cu2���� ��
��
���ð�ɫ��ĩ��������ʵ�飬�۲쵽���������£�
| ʵ����� | ���� |
| a.ȡ������ĩ����ˮ���� | ȫ���ܽ⡢ |
| ��Һ��ɫ�� | |
| b.��������Һ�����������������Һ�������� | ���������� |
| c.ȡ������ĩ�������� | ���������� |
| d.ȡ������ĩ����ϡH2SO4��ϡHNO3�Ļ��Һ | �а�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ŵ�����Ⱦ���������أ��������ڡ�ʮ�������ڼ䣬����������(SO2)�ŷ�������8%����������(NOx)�ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����
��1����CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)+4NO2(g) ="4NO(g)" + CO2(g) +2H2O(g) �SH=" -574" kJ��mol��1
��CH4(g) +4NO(g) =2N2(g) + CO2(g) + 2H2O(g) �SH=" -1160" kJ��mol��1
��H2O(g) = H2O(l) ��H=" -44.0" kJ��mol��1
д��CH4(g)��NO2(g)��Ӧ����N2 (g)��CO2 (g)��H2O(1)���Ȼ�ѧ����ʽ ��
��2������Fe2+��Fe3+�Ĵ����ã������¿ɽ�SO2ת��ΪSO42-���Ӷ�ʵ�ֶ�SO2����������֪��SO2�ķ���ͨ�뺬Fe2+��Fe3+����Һʱ������һ����Ӧ�����ӷ���ʽΪ4Fe2+ + O2+ 4H+ = 4Fe3+ + 2H2O������һ��Ӧ�����ӷ���ʽΪ ��
Ũ��/mol��L��1
| NO | N2 | CO2 | ||
| 0 | 1.00 | 0 | 0 | ||
| 10 | 0.58 | 0.21 | 0.21 | ||
| 20 | 0.40 | 0.30 | 0.30 | ||
| 30 | 0.40 | 0.30 | 0.30 | ||
| 40 | 0.32 | 0.34 | 0.17 | ||
| 50 | 0.32 | 0.34 | 0.17 |
 N2 (g)+CO2 (g) ��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2 (g)+CO2 (g) ��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪Ca(OH)2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ(�����ķ�Ӧ��Ϊ���ȷ�Ӧ)���������к���Cl����ClO����ClO3�����ֺ���Ԫ�ص����ӣ�����ClO����ClO3���������ӵ����ʵ���(n)�뷴Ӧʱ��(t)��������ͼ��ʾ��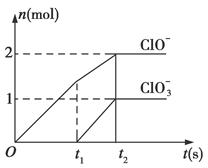
(1)t1ǰ������������______________________(�ѧʽ)��
(2)t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽ��_____________________________��
(3)��ʯ�����к���Ca(OH)2�����ʵ�����________mol��
(4)NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ��������________(����ĸ)��
| A��NaCl��Cl2 | B��NaCl��NaClO | C��NaClO3��NaClO4 | D��NaCl��NaClO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪�������ԣ�KMnO4��HNO3��Biλ�����ڱ��Т�A�壬��3�۽��ȶ���Bi2O3Ϊ���������Bi3������ҺΪ��ɫ��ȡһ���������ữ��Mn(NO3)2��Һ���ν�������ʵ�飬�����¼���£�
�������м���������NaBiO3����Һ��Ϊ�Ϻ�ɫ���ڼ����μ�����H2O2���Ϻ�ɫ��ȥ���������ݲ��������ټ���������PbO2���壬�����ܽ⣬��Һ�ֱ�Ϊ�Ϻ�ɫ��
�ش��������⣺
��1��д��ʵ��ٷ�Ӧ�����ӷ���ʽ��________________________________________��
��2��KMnO4��H2O2��PbO2��������ǿ������˳��Ϊ____________________________��
��3����Ӧ�۵õ�����Һ��ͨ��SO2���壬������������
________________________________________________________________________��
��4����ʵ��ڷų���336 mL����(��״��)����Ӧ�ٱ�������Mn(NO3)2Ϊ________ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���÷����ú��ʯ(��Ҫ��Al2O3��SiO2������������)�Ʊ���ˮ��BAC[Al2(OH)nCl6��n]���������£�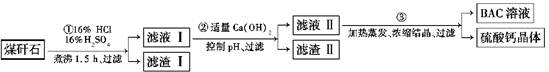
(1)����ú��ʯ��Ŀ����__________________________________________________��
���������Ҫ�ɷ���________(�ѧʽ)��
(2)���������еĹ����У���Һ������ɫ��Ϊ��ɫ����ʱ��Һ�е���ɫ����Ϊ_____
________(�ѧʽ)�������Һ�ֱ�Ϊ�ػ�ɫ����ط�Ӧ�����ӷ���ʽΪ_________������ٵ����װ���Ϸ��谲װһ�����ܣ������ܵ�������__________________________��
(3)������м���������Ca(OH)2������pH����Ŀ�ģ�һ������BAC������________
_______________________����֪BAC�ķ�ɢ�����Ӵ�С��1��100 nm֮�䣬�ɴ�������Һ����BAC����Һ�������������________________________����Ca(OH)2��Һ����������۵õ���BAC����ƫ�ͣ�д���÷�Ӧ�����ӷ���ʽ��_______________________
(4)��0.1 mol AlCl3��ij�¶�����������ˮ������5%ˮ������Al(OH)3��Һʱ����������a kJ��д���ù��̵��Ȼ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.���ǵؿ��к�����ߵĽ���Ԫ��,�䵥�ʼ���Ͻ������������е�Ӧ��ʮ�ֹ㷺��
(1)����������������Al2O3Ϊԭ��,�����ʯ(Na3AlF6)������״̬�½��е��,��ѧ����ʽΪ ����
��缫����ʯī��������,����ʱ�������ĵĵ缫����������(���������������)��
(2)������Ʒ���п���ʴ����,���ӳ���ʹ���������Դ�����������Ϊ����,��H2SO4��Һ�е��,���ı����γ�����Ĥ,������ӦʽΪ�� ��
(3)�����������Խ,Al-Ag2O��ؿ�����ˮ�¶�����Դ,��ѧ��ӦΪ:2Al+3Ag2O+2NaOH+3H2O 2Na[Al(OH)4]+6Ag, ���ĵ缫��ӦʽΪ
2Na[Al(OH)4]+6Ag, ���ĵ缫��ӦʽΪ
��������������������,����������Һ��pH��������(���������䡱��С��)��
��.���ǵ����Ϻ����ḻ��һ��Ԫ��,�����仯�����ڹ�ũҵ������������������Ҫ���á�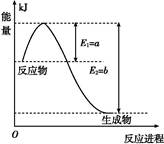
(1)��ͼ����һ���¶Ⱥ�ѹǿ��N2��H2��Ӧ����1 mol NH3�����������仯ʾ��ͼ,��д���ϳɰ����Ȼ�ѧ��Ӧ����ʽ: (��H����ֵ�ú���ĸa��b�Ĵ���ʽ��ʾ)��
(2)��ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g) 2NH3(g)����һ���¶���,��һ������N2��H2ͨ�����Ϊ1 L���ܱ�������,��Ӧ�ﵽƽ���,�ı���������,��ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ���������������
2NH3(g)����һ���¶���,��һ������N2��H2ͨ�����Ϊ1 L���ܱ�������,��Ӧ�ﵽƽ���,�ı���������,��ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ���������������
| A������ѹǿ | B������Ӧ���Ũ�� |
| C��ʹ�ô��� | D�������¶� |
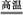 6SO2+Fe3O4,��3 mol FeS2�μӷ�Ӧ,ת����������mol���ӡ�
6SO2+Fe3O4,��3 mol FeS2�μӷ�Ӧ,ת����������mol���ӡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijУͬѧΪ̽��Br2��I2��Fe3����������ǿ��������������ʵ�顣
ʵ��٣�ȡ����KI��Һ���Թ��У��ȼ�����ˮ�����ټ���CCl4�����ã��۲쵽�²�Һ����Ϻ�ɫ��
ʵ��ڣ�ȡ����FeSO4��Һ���Թ��У��ȼ�����ˮ�����ټ����μ�����KSCN��Һ�����۲쵽��Һ�ʺ�ɫ��
��1��д�����ӷ���ʽ��
ʵ��٣�______________________________________________________��
ʵ��ڣ�______________________________________________________��
��2������������ʵ�飬�����ʵ������Կ��Եó�����ȷ������________��
| A��Br2>I2 | B��Fe3��>Br2 | C��Br2>Fe3�� | D��I��>Br�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D���ֿ������Σ��ֱ���Na+��Ba2+��Cu2+��Ag+��CO32����SO42����Cl����NO3���еIJ�ͬ�����Ӻ������Ӹ�һ����ɡ���������ʵ�飺
�� �������θ�ȡ�������ֱ�����ʢ��5 mL����ˮ����֧�Թ��У�ֻ��B����Һ����ɫ��
�� �ֱ���4֧�Թ��м���2 mLϡ���ᣬ����A����Һ�в�����ɫ������C����Һ���н϶����ݲ�������D����Һ����������
����������ʵ���ƶ��������εĻ�ѧʽ�ֱ�Ϊ��
A B C D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com