
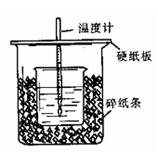
| �¶� ��� | ��ʼ�¶�t1/�� | ��ֹ�¶� T2/�� | �¶Ȳ� ��t/�� | ||
| HCl | NaOH | ƽ��ֵ | |||
| 1 | 25 | 25 | | 27.3 | |
| 2 | 25 | 25 | | 27.4 | |
| 3 | 25 | 25 | | 28.6 | |


 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1,3-������ϩ�ȱ��ȶ� |
| B������1,3-������ϩ�ȶ� |
| C��1,3-������ϩ���������ȷ�Ӧ |
| D�����������ӳ����ɻ����������ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
|
 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��K= �� ��������������С�������λ���֣�
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��K= �� ��������������С�������λ���֣� 
 N2(g)+O2(g)Ϊ(����ȡ����ȡ�) �� ��Ӧ��
N2(g)+O2(g)Ϊ(����ȡ����ȡ�) �� ��Ӧ�� N2(g)+O2(g) �Ѵﵽƽ����ǣ�����ţ��� ��
N2(g)+O2(g) �Ѵﵽƽ����ǣ�����ţ��� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��˸�ͬѧ�ó����ۡ�ú̿ȼ��ʱ������ˮ����ʹú̿ȼ�շų������������������֪ú̿��ȼ����Ϊ��393.15 kJ/mol��������ȼ����Ϊ��242 kJ/mol��һ����̼��ȼ����Ϊ��283 kJ/mol��
��˸�ͬѧ�ó����ۡ�ú̿ȼ��ʱ������ˮ����ʹú̿ȼ�շų������������������֪ú̿��ȼ����Ϊ��393.15 kJ/mol��������ȼ����Ϊ��242 kJ/mol��һ����̼��ȼ����Ϊ��283 kJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���κλ�ѧ��Ӧ�������������ı仯 |
| B��1 mol H2SO4�� 1 mol Ba(OH)2��ȫ��Ӧ���ų���������Ϊ�к��� |
| C����101kPa ʱ��1 mol ̼ȼ�շų�����������̼��ȼ���� |
| D���ڻ�ѧ��Ӧ����Ҫ���ȵķ�Ӧ�������ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A���÷�Ӧ�����ȷ�Ӧ |
| B��ʹ�ô�����Ӧ�ȼ�С |
C���Ȼ�ѧ����ʽΪCO(g)+2H2(g) �� CH3OH(g)��H��-510 kJ��mol��1  |
| D������a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾʹ�ô�����������仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ӧ��������������������������ʱ���÷�Ӧһ�����ܷ��� |
| B��ǿ���ǿ�Ӧ�ų������������к��� |
C����ʯī�Ƚ��ʯ�ȶ���֪�� |
D���� �� �� ʱ�� ʱ�� ��ȫȼ��������̬ˮ���ų� ��ȫȼ��������̬ˮ���ų� ��������������ȼ����Ϊ241.8 ��������������ȼ����Ϊ241.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


 ��
�� ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��A����Ȳ��ȼ����Ϊ |
B����ת�� ���ӣ������� ���ӣ������� |
C��������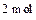 Һ̬ˮ���� Һ̬ˮ���� |
D�����γ� ̼�����õ���ʱ����ų�������Ϊ ̼�����õ���ʱ����ų�������Ϊ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

 HBr+H�������Է�Ӧ���̵�ʾ��ͼ��������������ȷ����
HBr+H�������Է�Ӧ���̵�ʾ��ͼ��������������ȷ����
| A������ӦΪ���ȷ�Ӧ |
| B��HBr����һ������H2������ |
| C������Ӧ������ѹǿ�Է�Ӧ����û��Ӱ�� |
| D���÷�Ӧ����ӦΪ���ܹ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com