
ЎѕМвДїЎїЅьДкАґОТ№ъїЖС§јТ·ўПЦБЛТ»ПµБРТвТеЦШґуµДМъПµі¬µјІДБПЈ¬ЖдЦРТ»АаОЄ FeЎЄS mЎЄAsЎЄFЎЄO ЧйіЙµД»ЇєПОпЎЈ»ШґрПВБРОКМвЈє
(1)ФЄЛШAsУлNН¬ЧеЎЈФ¤ІвAsµДЗв»ЇОп·ЦЧУµДБўМеЅб№№ОЄ____Ј¬ЖдЗв»ЇОп·Рµг±ИNH3µД__________(МоЎ°ёЯЎ±»тЎ°µНЎ±)Ј¬ ЖдЕР¶ПАнУЙКЗ________________ ЎЈ
(2)FeіЙОЄСфАлЧУК±КЧПИК§ИҐ______№мµАµзЧУЈ¬SmµДјЫІгµзЧУЕЕІјКЅОЄ4f66s2Ј¬Sm3+јЫІгµзЧУЕЕІјКЅОЄ____________ЎЈ
(3)Т»ЦЦЛД·ЅЅб№№µДі¬µј»ЇєПОпµДѕ§°ыИзНј 1 ЛщКѕЎЈѕ§°ыЦР Sm єН As ФЧУµДН¶У°О»ЦГИзНј2ЛщКѕЎЈНјЦР F- єН O2-№ІН¬ХјѕЭѕ§°ыµДЙППВµЧГжО»ЦГЈ¬ ИфБЅХЯµД±ИАэТАґОУГx єН 1ЎЄ x ґъ±нЈ¬ФтёГ»ЇєПОпµД»ЇС§КЅ±нКѕОЄ___________Ј»НЁ№эІв¶ЁГЬ¶И¦СєНѕ§°ыІОКэЈ¬їЙТФјЖЛгёГОпЦКµД x ЦµЈ¬НкіЙЛьГЗ№ШПµ±нґпКЅЈє¦С=_______gcm-3ЎЈ
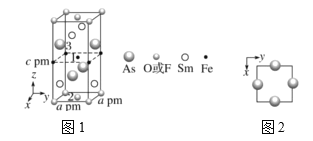
Ўѕґр°ёЎїИэЅЗЧ¶РО µН NH3·ЦЧУјдґжФЪЗвјь 4s 4f5 SmFeAsO1-xFx ![]()
ЎѕЅвОцЎї
(1) NH3µДБўМеЅб№№ОЄИэЅЗЧ¶РОЈ¬ФЄЛШAsУлNН¬ЧеЈ¬ФтAsµДЗв»ЇОпAsH3·ЦЧУµДБўМеЅб№№ТІОЄИэЅЗЧ¶РОЈ¬NH3·ЦЧУјдДЬРОіЙЗвјьЈ¬AsH3·ЦЧУјдІ»ДЬРОіЙЗвјьЈ¬ФтAsH3·Рµг±ИNH3µДµНЈ¬ЖдЕР¶ПАнУЙКЗNH3·ЦЧУјдґжФЪЗвјьЈ»
(2)FeјЫІгµзЧУЕЕІјКЅОЄ3d64s2Ј¬іЙОЄСфАлЧУК±,ТЧК§ИҐЧоНвІгµДµзЧУЈ¬ФтКЧПИК§ИҐ4s№мµАµзЧУЈ¬SmµДјЫІгµзЧУЕЕІјКЅОЄ4f66s2Ј¬Sm3+К§ИҐБЛИэёцµзЧУЈ¬ПИК§ИҐЧоНвІгБЅёцЈ¬ФЩК§ИҐ6fµДТ»ёцЈ¬ФтSm3+јЫІгµзЧУЕЕІјКЅОЄ4f5Ј»
(3)УЙНј1їЙЦЄЈ¬ѕ§°ыЦРSmФЧУУР4ёцФЪГжРДЈ¬FeФЧУУР4ёцФЪАвЙПєН1ёцФЪМеРДЈ¬AsФЧУУР4ёцФЪГжРДЈ¬F- єН O2-УР8ёцФЪ¶ҐµгєН2ёцГжРДЈ¬ёщѕЭѕщМЇ·ЁЈ¬Фтє¬SmФЧУЈє![]() Ј¬є¬FeФЧУЈє
Ј¬є¬FeФЧУЈє![]() Ј¬є¬AsФЧУЈє
Ј¬є¬AsФЧУЈє![]() Ј¬є¬F- Ўў O2-№ІЈє
Ј¬є¬F- Ўў O2-№ІЈє![]() Ј¬O2-УР2(1-x)ёцЈ¬F-УР2xЈ¬ЛщТФёГ»ЇєПОпµД»ЇС§КЅ±нКѕОЄSmFeAsO1-xFxЈ»ёщѕЭ»ЇС§КЅSmFeAsO1-xFxЈ¬Т»ёцѕ§°ыµДЦКБїОЄ
Ј¬O2-УР2(1-x)ёцЈ¬F-УР2xЈ¬ЛщТФёГ»ЇєПОпµД»ЇС§КЅ±нКѕОЄSmFeAsO1-xFxЈ»ёщѕЭ»ЇС§КЅSmFeAsO1-xFxЈ¬Т»ёцѕ§°ыµДЦКБїОЄ![]() Ј¬Т»ёцѕ§°ыµДМе»эОЄa2cЎБ10-30cm3Ј¬ФтГЬ¶И
Ј¬Т»ёцѕ§°ыµДМе»эОЄa2cЎБ10-30cm3Ј¬ФтГЬ¶И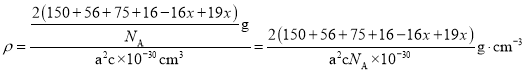


 єм№ыЧУИэј¶ІвКФѕнПµБРґр°ё
єм№ыЧУИэј¶ІвКФѕнПµБРґр°ё їОМГБ·јУІвПµБРґр°ё
їОМГБ·јУІвПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
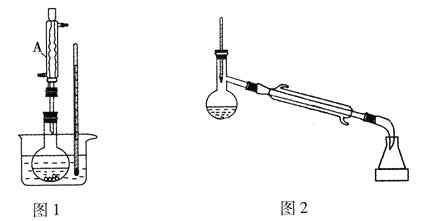
ЎѕМвДїЎїіЈОВПВ¶ФјЧ»щ±ЅјЧГСОЄОЮЙ«ТєМеЈ¬ѕЯУРЧПВЮАјµДПгЖшЈ¬їЙУЙ¶ФјЧ»щ±Ѕ·УУлјЧґјФЪЕЁБтЛбґЯ»ЇЧчУГПВЦЖИЎЈ¬·ґУ¦Ч°ЦГ(Ії·ЦјРіЦЧ°ЦГТСВФИҐ)ИзНјЛщКѕЈє
ЦЖ±ё·ґУ¦ОЄ![]() +CH3OH
+CH3OH![]()
![]() +H2OЎЈїЙДЬУГµЅµДКэѕЭИзПВЈє
+H2OЎЈїЙДЬУГµЅµДКэѕЭИзПВЈє
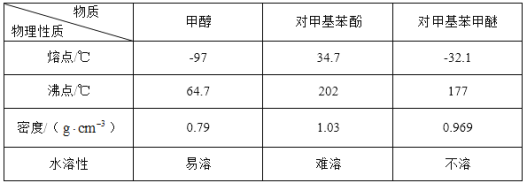
ўс.єПіЙ·ґУ¦ЈєФЪНј1µДЙХЖїЦРПИјУИлјёЖ¬ЛйґЙЖ¬Ј¬ФЩТАґОјУИл10.8g¶ФјЧ»щ±Ѕ·УЎў10mLјЧґјЈ¬ЧоєујУИл2mLЕЁБтЛбЈ¬їШЦЖ·ґУ¦ОВ¶ИОЄ60Ўж(Л®ФЎјУИИ)ЅшРР·ґУ¦ЎЈ
ўт.ІъОпМбґїЈєўЩЅ«·ґУ¦»мєПТєАдИґєујУИлЧгБї±ҐєНМјЛбДЖИЬТєЈ¬ід·Ц·ґУ¦єуЧЄТЖЦБ·ЦТєВ©¶·ЦРЈ¬ѕІЦГ·ЦТєЈ»ўЪЅ«УР»ъІгЧЄТЖЦБНј2ЙХЖїЦРЈ¬јУИИЈ¬їШЦЖОВ¶ИОЄ100ЎжЅшРРХфБ󣬴эХфБуЅбКшєуЈ¬ПтЙХЖїДЪКЈУаТєМеЦРјУИлЧгБїОЮЛ®ВИ»ЇёЖЈ¬И»єуіГИИ№эВЛЈ¬ІўЅ«ЛщµГТєМеФЩґОЅшРРХфБуЈ¬КХјЇ177ЎжЧуУТБу·ЦЈ¬ХфБуЅбКшєуЈ¬іЖБїЛщµГБу·ЦОЄ7.32gЎЈ
»ШґрПВБРОКМвЈє
ЈЁ1Ј©ТЗЖчAµДГыіЖОЄ__ЎЈ
ЈЁ2Ј©ПтНј1µДЙХЖїЦРјУИлЛйґЙЖ¬µДДїµДКЗ__Ј»ІЙУГЛ®ФЎјУИИµДДїµДКЗ__ЎЈ
ЈЁ3Ј©ІъОпМбґїК±Ј¬Пт·ґУ¦»мєПТєЦРјУИлЧгБї±ҐєНМјЛбДЖИЬТєµДЧчУГКЗ__Ј»ІъОпМбґїК±Ј¬ПИїШЦЖОВ¶ИОЄ100ЎжЅшРРХфБуµДДїµДКЗ__ЎЈ
ЈЁ4Ј©јУИлЧгБїОЮЛ®ВИ»ЇёЖµДДїµДКЗ__ЎЈ
ЈЁ5Ј©КХјЇµЅµД177ЎжЧуУТµДБу·ЦЦчТЄКЗ__(МоГыіЖ)ЎЈ
ЈЁ6Ј©¶ФјЧ»щ±ЅјЧГСµДІъВКОЄ__ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїЗлФЪГїёц»ЇС§·ЅіМКЅєуµДєбПЯЙПЧўГч»ЇС§·ґУ¦№эіМЦРДЬБїµДЦчТЄЧЄ»ЇРОКЅЎЈ
(1)Zn+Ag2O+H2O![]() Zn(OH)2+2AgЈє______(МоХэПт·ґУ¦µДДЬБїЧЄ»ЇРОКЅ)ЎЈ
Zn(OH)2+2AgЈє______(МоХэПт·ґУ¦µДДЬБїЧЄ»ЇРОКЅ)ЎЈ
(2)2C2H2+5O2![]() 4CO2+2H2OЈє______ЎЈ
4CO2+2H2OЈє______ЎЈ
(3)6CO2+6H2O![]() C6H12O6+6O2Јє______ЎЈ
C6H12O6+6O2Јє______ЎЈ
(4)CaCO3![]() CaO+CO2Ўь______ЎЈ
CaO+CO2Ўь______ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїПВБР·ґУ¦ЦРЈ¬Na2O2Ц»±нПЦЗїСх»ЇРФµДКЗ
A. 2Na2O2 + 2CO22Na2CO3 + O2
B. Na2O2 + MnO2 =Na2MnO4
C. 5Na2O2 + 2MnO![]() + 16H+ = 10Na+ + 2Mn2+ + 5O2Ўь + 8H2O
+ 16H+ = 10Na+ + 2Mn2+ + 5O2Ўь + 8H2O
D. 2Na2O2 + 2H2SO4 = 2Na2SO4 +2H2O + O2Ўь
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїУР»ъ»ЇєПОпM КЗєПіЙДіЦЦї№РВ№Ъ·ОСЧТ©ОпµДЦРјдМеЎЈM µДЅб№№јтКЅИзНјЛщКѕ ЎЈПВБРУР№ШM µДµДРрКцХэИ·µДКЗ
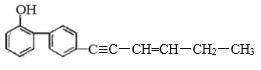
A.ёГУР»ъОпµД·ЦЧУКЅОЄ C18H15O
B.lmolM Чо¶аДЬУл 5molBr2 (ЛДВИ»ЇМјИЬТє)·ўЙъ·ґУ¦
C.M ·ЦЧУЦРЦБЙЩУР11 ёцМјФЧУ№ІЖЅГж
D.M ·ЦЧУЦРЦБЙЩУР5ёцМјФЧУФЪТ»МхЦ±ПЯЙП
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎї(1)УРПВБР»ЇєПОпЈєјЧ Јє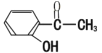 ТТЈє
ТТЈє ![]() ±ыЈє
±ыЈє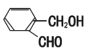 ¶ЎЈє
¶ЎЈє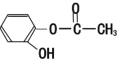
ўЩЗл°ґЛбРФУЙЗїЦБИхЕЕБРјЧЎўТТЎў±ыµДЛіРтЈє________(УГјЧТТ±ы±нКѕ)
ўЪРґіцТТУлТТґј·ўЙъхҐ»Ї·ґУ¦µД»ЇС§·ЅіМКЅЈє____________ЎЈ
(2)УЙ±ыП©ПВБР·ґУ¦їЙЦЖµГFЎўGБЅЦЦёЯ·ЦЧУ»ЇєПОпЈ¬ЛьГЗ¶јКЗіЈУГµДЛЬБПЎЈ
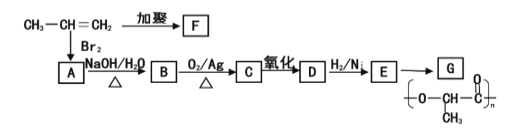
ўЩРґіцEµДЅб№№јтКЅЈє_______ЎЈ
ўЪCУлРВЦЖµДCu (OH) 2 №ІИИЧЄ»ЇОЄDµД»ЇС§Б¦іМКЅКЗЈє________ЎЈ
ўЫРґіц±ыП©ЎъFµД»ЇС§·ЅіМКЅ________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїТСЦЄ25 ЎжЎў101 kPaК±Ј¬1 mol H2УлдеХфЖшНкИ«·ґУ¦ЙъіЙЖшМ¬де»ЇЗв·ЕіцДЬБїQ kJЈ¬ФтПВБРИИ»ЇС§·ЅіМКЅКйРґХэИ·µДКЗ (ЎЎЎЎ)ЎЈ
A.H2(g)Ј«Br2(g)=2HBr(g) ¦¤HЈЅЈ2Q kJЎ¤molЈ1
B.H2(g)Ј«Br2(l)=2HBr(g)ЎЎ ¦¤HЈЅЈQ kJЎ¤molЈ1
C.![]() H2(g)Ј«
H2(g)Ј«![]() Br2(g)=HBr(g)¦¤HЈЅЈ«
Br2(g)=HBr(g)¦¤HЈЅЈ«![]() kJЎ¤molЈ1
kJЎ¤molЈ1
D.HBr(g)=![]() H2(g)Ј«
H2(g)Ј«![]() Br2(g)¦¤HЈЅЈ«
Br2(g)¦¤HЈЅЈ«![]() kJЎ¤molЈ1
kJЎ¤molЈ1
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїXЎўYЎўZЎўWУРИзНјЛщКѕµДЧЄ»Ї№ШПµЈє

ЈЁ1Ј©ИфXµҐЦКµДТ»ЦЦН¬ЛШТмРОМеКЗТ»ЦЦєЪЙ«µД·ЗЅрКфµҐЦКЈ¬ФтYЧЄ»ЇОЄZµД»ЇС§·ЅіМКЅ_________________Ј¬ZµДѕ§МеАаРН______________ѕ§МеЎЈ
ЈЁ2Ј©ИфXОЄТ»ЦЦЅрКфµДВИ»ЇОпЈ¬YКЗТ»ЦЦіЈјыµДБЅРФЗвСх»ЇОпЈ¬WОЄ»Ї№¤іЈУГµДЗїјоЈ¬РґіцYУлW·ґУ¦µДАлЧУ·ЅіМКЅ_______________________ЎЈ
ЈЁ3Ј©ИфXКЗТ»ЦЦ»оЖГµДЅрКфµҐЦКЈ¬ZКЗТ»ЦЦµ»ЖЙ«µД»ЇєПОпЈ¬ZµД»ЇС§КЅ___________Ј¬ФтZЧЄ»ЇОЄWµД»ЇС§·ЅіМКЅ________________________________________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїПВБРАлЧУ·ЅіМКЅКйРґХэИ·µДКЗ![]()
A.ЅрКфДЖН¶ИлЛ®ЦРЈє![]()
B.НщЛбРФµв»ЇјШИЬТєЦРµОјУККБїµДЛ«СхЛ®Јє![]()
C.Нщ![]() ИЬТєЦРјУ№эБїµДNaOHИЬТєІўјУИИЈє
ИЬТєЦРјУ№эБїµДNaOHИЬТєІўјУИИЈє![]()
![]()
![]()
D.ВИЖшНЁИлЛ®ЦРЈє![]()
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com