
��14�֣���ҵ��SnSO4��һ����Ҫ�������Σ��㷺Ӧ���ڶ�����ҵ�����Ʊ�·�����£�
��ʾ������֪�����������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��
����֪Ksp[Sn(OH)2] ��1.0��10-26
�ش��������⣺
��1��SnCl2�����������ˮֱ���ܽ��ԭ����__________������Sn�۵�������_________��
��2����ӦI���ɵij���ΪSnO��д���÷�Ӧ�����ӷ���ʽ___________________________��
��3����������Ѿ���Ưϴ���ɾ��ķ���__________________________________________��
��4����Ӧ�����������֮һ�ǿ�����Һ��pH������Һ��c(Sn2+)��1.0mol?L-1����Ӧ������ҺpH_____��
��5�����������£�SnSO4����������˫��ˮȥ��������д��������Ӧ�����ӷ���ʽ____________��
��1������Sn2+ˮ�� ��2�֣� ��ֹSn2+��������2�֣���2��Sn2+ + CO32-��SnO�� + CO2����3�֣�
��3��ȡ���һ��ϴ��Һ�������м���AgNO3��Һ������������˵��ϴ�Ӹɾ�����2�֣�
��4��С��1�� ��2�֣� ��5��Sn2+ + H2O2 + 2H+��Sn4+ + 2H2O ��3�֣�
���������������1��SnCl2��ˮ�����ɼ�ʽ�Ȼ�����������ƽ��Sn Cl2+H2O Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
��2����Ӧ��õ�������SnO��SnԪ�ػ��ϼ�û�б仯�����ڷ�������ԭ��Ӧ��ͬʱ�������壬������Ϊ������̼�����ӷ���ʽΪ��Sn2++CO32-�TSnO��+CO2����
��3�����������������������ӣ���˼�������Ѿ���Ưϴ���ɾ��ķ����ǣ�ȡ���һ��ϴ��Һ�������м���AgNO3��Һ������������˵��ϴ�Ӹɾ���
��4������ Ksp[Sn��OH��2]��1.0��10-26��c��OH-��2��c��Sn2+������c��Sn2+����1.0mol?L-1���˿ɵ�c��OH-����10-13mol/L��c��H+����0.1mol/L����pHС��1��Sn2+��ȫ������
��5�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ�Sn2+�ױ�����ΪSn4+����������ԭΪˮ�����ӷ���ʽΪ��Sn2++H2O2+2H+�TSn4++2H2O��
���㣺����Թ������̵����⡢���ʵķ����ᴿ���Ķ���Ŀ��ȡ��Ϣ�����������û�ѧ������д�����ù�ϵʽ���еļ����


 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ʵ��װ�úͷ���������Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ��� 
ͼ1 ͼ2 ͼ3 ͼ4
| A����ͼ1��ʾװ�÷����Ҵ������� |
| B����ͼ2��ʾ��װ��������ƿ��ת��Һ�� |
| C����ͼ3��ʾ��װ���Ʊ��������� |
| D����ͼ4��ʾ��װ�÷���ʯ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪�ס��ҵ�ijЩ�������£�
| ���� | �ܶ�/(g��mL��1) | �е� | �ܽ��� |
| �� | 0.893 | 78.5�� | ������ˮ�������� |
| �� | 1.220 | 100.7�� | ������ˮ�����ڼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ���ۺ����ÿ����Ʊ�����þ������������ͼ��ʾ��
��ʾ����MgCl2����Ļ�ѧʽΪMgCl2��6H2O��
��MgO���۵�Ϊ2852oC����ˮMgCl2���۵�Ϊ714oC��
��1�������ڵ�������________��________�����ˡ�
��2���Լ�a��������_______________________��
��3��Mg(OH)2�����л��е�Ca(OH)2Ӧ������ȥ��д��ʵ�鲽��_____________________________
_________________________________________________________________________��
��4����ҵ���ǽ�������þת��Ϊ�Ȼ�þ���ٵ���Ȼ�þ����ȡþ���ʣ��������ã�����Mg(OH)2�õ�MgO���ٵ������MgO�ķ����ƽ���þ����ԭ����_______________________________��
��5��ʵ�����ォ�����Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ����������ֱ�˵�����������ʹ�ò�������Ŀ�ģ�
����ʱ��__________________________������ʱ��________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣��Ͼɼ���п�̸ɵ���ڲ��ĺ�ɫ����A��Ҫ����MnO2��NH4CI��ZnCI2������������FeCI2��̿�ۣ���A�Ʊ��ߴ�MnCO3��������ͼ���¡�
��1������п�̸ɵ�صĸ���������_________(�ѧʽ)��
��2���ڢ�����Ŀ����________________________��
��3���ڢ��������Ƕ���Һa������ȳ��ӣ���ȥZn2+�����ӷ���ʽΪ____________________��
���� (��֪��Ksp(MnS)=2.5��10-13��Ksp(ZnS)=1.6��10-24)
��4��Ϊѡ���Լ�X������ͬ�����£��ֱ���5 g��ɫ����M�����Ʊ�MnSO3��ʵ�飬�õ��������ұ���
�����Լ�x�����ѡ����_________��
���ڵڢ�����ѡ��������Լ�X��M����Ҫ�ɷַ�Ӧ�Ļ�ѧ����ʽΪ_________��
��5����֪��MnCO3������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻Mn(OH)2��ʼ����ʱpHΪ7.7���벹��������²�����
�ڢ���ϵ�в����ɰ�һ�����̽��У��벹����ɲ��������ڢ���ϵ�в����пɹ�ѡ�õ��Լ��� ���Ҵ���
���Ҵ���
����1��___________________������2�����ˣ�������ˮϴ��2��3��
����3�������Һ������SO42-�ѳ��ɾ���������4��___________________��
����5�����º�ɡ�
��6������1���ܷ�����Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ������������ຬ��Cu��Au���𣩺�PbSO4�����ʣ�ʪ����������������ۺ����õ��������£�
��1����CuSO4�����Һ��⺬ͭ����Ǧ�Ĵ�ͭ�������ĵ缫��Ӧʽ�У� ��Cu-2e-= Cu2+��
��2������������ʱ��Ϊ����߱���Ч�ʣ���ȡ�ĺ�����ʩ�� �����պ���������г�����PbSO4�⣬���� ���ѧʽ����
��3������I����Ҫ����Ϊ ��������������� ��
��4��д����SO2��ԭAuCl4-�����ӷ���ʽ ��
��5��Ϊ�˼��ٷ�Һ�ŷš��������������Դ����ҵ�Ͻ���Һ1��������ͭ��Һ����ѭ����������ָ������ͼ����һ�����Ƶ����� ��
��6����֪298Kʱ��Ksp(PbCO3)=1.46��10-13��Ksp(PbSO4)= 1.82��10-8�������ӷ���ʽ��ʾ����̼������Һ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ� Fe2����Mg2����Al3����Ba2���� ��
�� ��Cl����I����
��Cl����I���� ��ȡ����Һ����ʵ�飺
��ȡ����Һ����ʵ�飺
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ��� |
| (2)ȡ��������Һ���ȣ���CuƬ��ŨH2SO4������ | ����ɫ���������������������ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ���� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | �а�ɫ�������Ҳ�����ϡHNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ������NaOH����ʱ���������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������SiO2��Al2O3�����ʣ��Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7���Ĺ�������ͼ��ʾ��
��֪���� NaFeO2��ˮǿ��ˮ�⡣
�� Cr2O72��+ H2O 2CrO42��+ 2H+
2CrO42��+ 2H+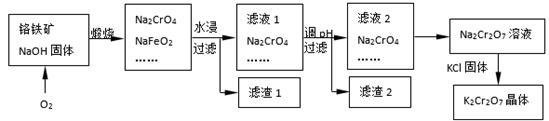
��1��K2Cr2O7��CrԪ�صĻ��ϼ��� ��
��2����Һ1�ijɷֳ�Na2CrO4��Na2SiO3�⣬�����У��ѧʽ�� ��
��������1�к��ɫ���ʷ�Ӧ�Ļ�ѧ����ʽ�ǡ�
��3������Һ2ת��ΪNa2Cr2O7��ҺӦ��ȡ�Ĵ�ʩ�ǡ�
��4�����ո���������Na2CrO4��NaFeO2��Ӧ�Ļ�ѧ����ʽ�� ��
��5��Ī������һ�ֳ����ζ�������Na2CrO4 Ϊָʾ�����ñ���������Һ�ζ�����Һ�����вⶨ��Һ��Cl����Ũ�ȡ���֪��
| ���� ���� | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ��ɫ | �� | dz�� | �� | ש�� | �� |
| Ksp | 1��34��10��6 | 7��1��10��7 | 1��1��10��8 | 6��5��10��5 | 1��0��10��6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�ִ����к�����ɳ��Ca2����Mg2����Fe3����SO�����ʡ�ijͬѧ��ʵ����������������ִ����Ʊ����εķ�������(���ڳ������Լ��Թ���)��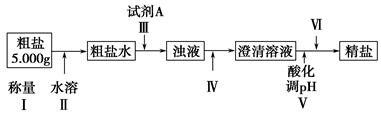
��ش��������⣺
(1)Ϊ������ѡ����������(�ñ����ĸ��д)��________��
A���ձ���B���Թܡ�C����������D����Һ©����E��©����F���ƾ��ơ�
G��������
(2)�������г���Na2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ϊ�����Լ������������Լ���˳��Ϊ��NaOH��Һ��________��________��
(3)�������У��жϼ���BaCl2�ѹ����ķ�����___________________________________
(4)������Ӧѡ�������________����������������������Ⱥ�˳��Ե��������ʵ����������Ӱ����___________________________________
(5)��������________(ѡ��������������ƣ��ñ����ĸ�������Ⱥ�˳����д)��
a�����ˡ�ϴ�� B��������Ũ�� c����ȡ����Һ D����ȴ���ᾧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com