
����Ŀ��25��ʱ����Ũ�Ⱦ�Ϊ0.1mol/L������ֱ�ΪVa��Vb��HA��Һ��BOH��Һ����ͬ����Ȼ�ϣ�����Va+Vb=100mL��Va��Vb����Һ��pH�Ĺ�ϵ��ͼ��ʾ������˵������ȷ����
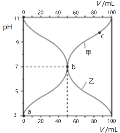
A.��c��Ӧ����Һ���У�c(B+)��c(BOH)��0.1molL��1
B.��b��Ӧ����Һ���У�c(B+)��c(A��)
C.���ױ�ʾ����BOH��Һ�������pH�Ĺ�ϵ����
D.���ɵ�a����c�Ĺ��̣�ˮ�ĵ���̶���������С
���𰸡�A
��������
��ͼ����Ϣ��֪��Va=100mLʱ��pH=3��c(H+)=1��10-3mol/L����HAΪ���Vb=100mLʱ��pH=11��c(OH-)=1��10-3mol/L����BOHΪ���������ʼ��pH=3����HA��Һ�м���BOH��Һ��pH���ߣ��������ױ�ʾ����BOH��Һ�������pH�Ĺ�ϵ���ߣ������ҵ���ʼ��pH=11����BOH��Һ�м���HA��Һ��pH���ͣ����������ұ�ʾ����HA��Һ�������pH�Ĺ�ϵ���ߣ��ݴ˷������
A����c��Ӧ����Һ��VamLHA��VbmL��BOH��Һ�Ļ����Һ��Va+Vb=100mL��Vb��Va����50mL��Vb��100mL������n(BA)��0.1mol/L��0.1L=0.01mol����n(B+)+n(BOH)��0.01mol���ʵ�c��Ӧ����Һ��c(B+)+c(BOH)=![]() ��
��![]() =0.1molL-1����A����
=0.1molL-1����A����
B����b��Ӧ����Һ�����ԣ�����ݵ���غ��֪��Һ���У�c(B+)=c(A-)����B��ȷ��
C����HA��Һ�м���BOH��Һ��pH���ߣ��������ױ�ʾ����BOH��Һ�������pH�Ĺ�ϵ���ߣ���C��ȷ��
D����������ˮ�ĵ��룬���ε�ˮ��ٽ�ˮ�ĵ��룬����a��c������ˮ�ĵ���̶���������С����D��ȷ��
��ѡA��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ����������ֶ�����Ԫ�ص�λ����ͼ��ʾ(�����ҡ���������λ��δ���)��
![]()
��֪����Ԫ�ص�ԭ������֮��Ϊ36���ҵ�ԭ��������ס���ԭ������֮����ȡ�
(1)��Ԫ�������ڱ��е�λ��Ϊ______________��
(2)���������γ�AB2�ͻ�����仯ѧʽΪ________��
(3)������Ȼ�����γɻ�������������Ԫ�أ������________(������)���䵥�����Ԫ�ص�����������Ӧˮ�����Ũ��Һ����ʱ��Ӧ�Ļ�ѧ����ʽ��______________________��
(4)���ס��ҡ�����������Ԫ����������ʱ��������������Ԫ�صõ��Ļ�����������࣬����________(��Ԫ�ط���)��д����Щ���������������ַ������������Ļ�ѧʽ______________________
(��ԭ�Ӹ�����Ϊ1��2���ڷ����м۵�������Ϊż��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���G��һ��ҩ���м��壬�ϳ� G�IJ����������£�

��ش��������⣺
��1��G�����еĺ��������ŵ�������___________��____________��
��2�������ݱ仯���̵ķ�Ӧ������_____________��
��3����ӦA��B�Ļ�ѧ����ʽΪ________________________________________��
��4��д����������������C��ͬ���칹��Ľṹ��ʽ��_______________________��
��. ������ֻ������ȡ������
��. ������ֻ��4�ֲ�ͬ��ѧ�������⡣
��. ����NaHCO3��Ӧ����CO2��
��5����������֪ʶ����������Ϣ��д����![]() ��������Ϊԭ���Ʊ�
��������Ϊԭ���Ʊ�![]() �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�H2C��CH2
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�H2C��CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2OH__________
CH3CH2OH__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������֮Ϊ������ء���ֽ�ʵ�أ������ܷ�ӦΪZn+2MnO2+H2O![]() ZnO+2MnO(OH)������˵����ȷ����( )
ZnO+2MnO(OH)������˵����ȷ����( )
A���õ�ص�����Ϊп
B���õ�ط�Ӧ�ж����������������
C����65 g Zn��ȫ�ܽ�ʱ�������缫�ĵ���Ϊ1mol
D�����������ӦʽΪ2MnO2+2e-+2H2O![]() 2MnO(OH)+2OH-
2MnO(OH)+2OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о��������ﷴӦ���������ڿ�������β����������������Ҫ���塣
��1��NO�ڿ����д������·�Ӧ��2NO(g) + O2(g) ![]() 2NO2(g) ��H��������Ӧ��������ɣ����е�һ����Ӧ�����£�д���ڶ�����Ӧ�ڵ��Ȼ�ѧ����ʽ���䷴Ӧ���ʱ䦤H2�ú���H����H1��ʽ������ʾ���� �� 2NO(g)
2NO2(g) ��H��������Ӧ��������ɣ����е�һ����Ӧ�����£�д���ڶ�����Ӧ�ڵ��Ȼ�ѧ����ʽ���䷴Ӧ���ʱ䦤H2�ú���H����H1��ʽ������ʾ���� �� 2NO(g) ![]() N2O2(g)��H1��0���� ___________��
N2O2(g)��H1��0���� ___________��
��2��NH3����ԭ��������(SCR)������ĿǰӦ����㷺���������������ѳ���������Ӧ���Ȼ�ѧ����ʽΪ�� 4NH3��g��+6NO��g��![]() 5N2��g��+6H2O��g����H=��1811.63KJ/mol����Ӧ�ں����ܱ������н��У�������������ͬʱ��ѡ�ò�ͬ�Ĵ�������Ӧ����N2�����ʵ�����ʱ��仯��ͼ��ʾ��
5N2��g��+6H2O��g����H=��1811.63KJ/mol����Ӧ�ں����ܱ������н��У�������������ͬʱ��ѡ�ò�ͬ�Ĵ�������Ӧ����N2�����ʵ�����ʱ��仯��ͼ��ʾ��

���ڴ���A�������£�������ͬʱ�䣬����ѵ����淴Ӧ�¶ȵı仯�����ͼ2��ʾ����ͼ��֪������ͬ��ʱ���ڣ�300��֮ǰ���¶������ѵ���������300��֮���¶������ѵ�����С��������ĩʧЧ����д��300��֮���ѵ��ʼ�С��ԭ����_________��
������������ͬʱ������ͼ�в����ڴ���B�������ѵ������¶ȱ仯������________��

��3����ҵ��HNO3��β���к��е�NO2��NO����NaOH��Һ���գ���Ӧ�Ļ�ѧ����ʽΪ��NO+NO2+2NaOH��2NaNO2+H2O��2NO2+2NaOH��NaNO2+NaNO3+H2O������ƽ�����ΪNOx��NO��NO2������壬ͨ��������NaOH��Һ�У���ַ�Ӧ��û������ʣ�࣬��
��x��ȡֵ��ΧΪ_________________��
�ڷ�Ӧ����Һ��n(NO2��)�Un(NO3��)=____________________��(�ú�x�Ĵ���ʽ��ʾ��
��4����ⷨ����������������Ŀǰ������Ⱦ������һ����˼·��ԭ���ǽ�NOx�ڵ����зֽ������Ⱦ��N2��O2��ȥ����ͼʾ�����缫�����������������մɣ���һ�������¿����ɴ���O2��������������ӦΪ___��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǵ�ѭ�������е���Ҫ���ʣ����ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������
��1��������ͼ�ṩ����Ϣ��д���÷�Ӧ���Ȼ�ѧ����ʽ___����ͼ��������__���a����b������ʾ��������ý���������������仯���ߡ�
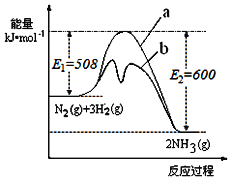
��2���ں��������У�������������˵��������Ӧ�Ѵ�ƽ�����___��
A��3��(H2)����2��(NH3)��
B����λʱ��������nmolN2��ͬʱ����2nmolNH3
C��������������ܶȲ���ʱ��ı仯���仯
D��������ѹǿ����ʱ��ı仯���仯
��3��500�桢50MPaʱ�����ݻ�Ϊ1L�������м���1molN2��3molH2����Ӧ��ƽ�����ƽ�ⳣ��ΪK����ʱN2��ת����Ϊa����K��a�Ĺ�ϵ��K��___��
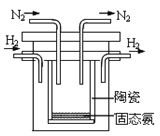
��4��1998��ϣ������˹��´�ѧ����λ��ѧ�Ҳ��ø����ӵ����Ե�SCY�մɣ��ܴ���H+����ʵ���˸��³�ѹ�¸�ת���ʵĵ��ϳɰ�����ʵ��װ����ͼ�������ĵ缫��ӦʽΪ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���£���6.72 LCH4����3.01��1023��HCl���ӣ���13.6 gH2S����0.2 molNH3�����ж������������к�����ԭ����Ŀ�Ӵ�С������ȷ���ǣ� ��
A.�٢ۢڢ�B.�٢ۢܢ�C.�ڢۢ٢�D.�ڢܢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������и�ͼ����������ȷ���ǣ� ��
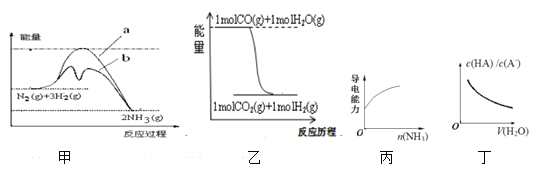
A.ͼ��������b��ʾ��ҵ�ϳɰ��м�������ý���������������仯����
B.����ͼ�ҿ�֪�ڸ������£�CO2(g)+H2 (g)=CO(g)+H2O(g) ��H<0
C.ͼ�������߱�ʾ��1.0mol��L��1�Ĵ�����Һ��ͨ�백������Һ���������ı仯���
D.ͼ�������߱�ʾ������������HA��ϡ��Һ�м�ˮϡ��ʱ��c(HA)/c(A��)�ı仯���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ijЩǿ���ε�ˮ��Һ�����ԣ���NaCl��Һ��ijЩ�����ε�ˮ��Һ�ʼ��ԣ���Na2CO3��Һ�����������ͼ��ʾת����ϵ�ش��й����⡣A��B��Ϊ��ɫ��Ӧ�ʻ�ɫ��ˮ��Һ��A�����ԣ�B�ʼ��Բ�����ǿ�����ԡ�
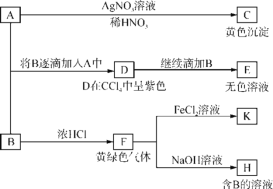
��1��д��C�Ļ�ѧʽ��________��
��2������д��A��D��D��E(E�к���ij��5��Ԫ�صĺ����������)�����ӷ���ʽ�� ___________________��_____________________��
��3��д����SO2����ͨ��K��Һ�з�����Ӧ�����ӷ���ʽ��____________��
��4������K��Һ�������ӵļ�������__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com