
����Ŀ��ͭ����Ҫ������Cu�Ļ������ڿ�ѧ�о���ҵ�����о���������;����CuSO4��Һ���������Һ�����Һ�ȡ���ش��������⣺
��1��CuSO4���ɽ���ͭ��Ũ���ᷴӦ�Ʊ����÷�Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________��
��2��CuSO4��ĩ����������һЩ�л����е���ˮ�֣���ԭ����________________________________________________________________________��
��3��SO![]() ��S��sp3�ӻ���SO
��S��sp3�ӻ���SO![]() �����幹����________��
�����幹����________��
��4��Ԫ�ؽ�(Au)�������ڱ��еĵ������ڣ���Cuͬ�壬��ԭ�����������Ų�ʽΪ____________��һ��ͭ�Ͻ�������������ܶѻ��Ľṹ���ھ�����ͭԭ�Ӵ������ģ���ԭ�Ӵ��ڶ���λ�ã���úϽ���ͭԭ�����ԭ������֮��Ϊ________���þ����У�ԭ��֮�����������________________________________________________________________________��
��5��CuSO4����Ĺ�������________��________���������������________���þ�������________���塣
��6������������д���ܣ���ԭ�ӿɽ��뵽��ͭԭ�����ԭ�ӹ��ɵ��������϶�С�����ͭԭ�����ԭ�ӵ�ͬ�������þ��崢���ľ����ṹ��CaF2�Ľṹ���ƣ��þ��崢���Ļ�ѧʽӦΪ________��
���𰸡� Cu��2H2SO4(Ũ)![]() CuSO4��SO2��ʮ��2H2O ��ɫCuSO4��ĩ��ˮ���������ɫ��CuSO4��5H2O���� ���������� 6s1 3��1 ������ Cu2�� SO
CuSO4��SO2��ʮ��2H2O ��ɫCuSO4��ĩ��ˮ���������ɫ��CuSO4��5H2O���� ���������� 6s1 3��1 ������ Cu2�� SO![]() ���Ӽ� ���� Cu3AuH8
���Ӽ� ���� Cu3AuH8
����������1��Cu��Ũ�����ڼ��������·�Ӧ���ɶ������������ͭ����Ӧ�ķ���ʽΪCu��2H2SO4(Ũ)![]() CuSO4��SO2��ʮ��2H2O����2����2��CuSO4��ĩ����������һЩ�л����е���ˮ�֣���ԭ���ǰ�ɫCuSO4��ĩ��ˮ���������ɫ��CuSO4��5H2O��������3�����������ԭ�ӵļ۲���Ӷ�Ϊ���¶Ե�����
CuSO4��SO2��ʮ��2H2O����2����2��CuSO4��ĩ����������һЩ�л����е���ˮ�֣���ԭ���ǰ�ɫCuSO4��ĩ��ˮ���������ɫ��CuSO4��5H2O��������3�����������ԭ�ӵļ۲���Ӷ�Ϊ���¶Ե�����![]() =0���ɼ����Ӷ���4������Ϊ��������ṹ����4��Ԫ�ؽ�Au���������ڱ��еĵ������ڣ���Cuͬ�壬������������Ϊ1�������������Ų�ʽΪ6s1���ھ�����Cuԭ�Ӵ������ģ�N��Cu��=6��
=0���ɼ����Ӷ���4������Ϊ��������ṹ����4��Ԫ�ؽ�Au���������ڱ��еĵ������ڣ���Cuͬ�壬������������Ϊ1�������������Ų�ʽΪ6s1���ھ�����Cuԭ�Ӵ������ģ�N��Cu��=6��![]() =3��Auԭ�Ӵ��ڶ���λ�ã�N��Au��=8��
=3��Auԭ�Ӵ��ڶ���λ�ã�N��Au��=8��![]() =1����úϽ���Cuԭ����Auԭ������֮��Ϊ3��1��Ϊ�������壬ԭ�Ӽ��������Ϊ����������������5��CuSO4����Ĺ�������Cu2����SO42-������������������Ӽ����þ����������Ӿ��壻��6��CaF2�Ľṹ��ͼ
=1����úϽ���Cuԭ����Auԭ������֮��Ϊ3��1��Ϊ�������壬ԭ�Ӽ��������Ϊ����������������5��CuSO4����Ĺ�������Cu2����SO42-������������������Ӽ����þ����������Ӿ��壻��6��CaF2�Ľṹ��ͼ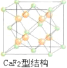 ����ԭ�ӿɽ��뵽��Cuԭ����Auԭ�ӹ��ɵ��������϶�У���Hԭ��Ӧλ�ھ����ڲ�����Ӧ����8��H����ѧʽΪCu3AuH8��
����ԭ�ӿɽ��뵽��Cuԭ����Auԭ�ӹ��ɵ��������϶�У���Hԭ��Ӧλ�ھ����ڲ�����Ӧ����8��H����ѧʽΪCu3AuH8��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС����ʵ������ȡ Na2O2���������Ͽ�֪����������� 453473K ֮������� Na2O��Ѹ������¶ȵ� 573673K ֮������� Na2O2�����¶���ߵ� 733873K ֮�� Na2O2 �ɷֽ⡣
(1)���������ȡ Na2O2 װ����ͼ1��
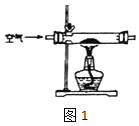
��ʹ�ø�װ����ȡ�� Na2O2 �п��ܺ��е�����Ϊ_____��.
A��NaCl B��Na2CO3 C��Na2O D��NaOH E��NaHCO3
�ڸ�С��Ϊ�ⶨ�Ƶõ� Na2O2 ��Ʒ�Ĵ��ȣ���ƿ����õ���װ����ͼ 2:��ƿ�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��_________���ⶨװ�õĽӿڴ� ��������ȷ������˳����_____��
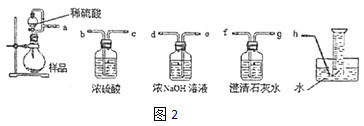
(2)����ӷ�Ӧ�����Ϸ����òⶨ��Ӧ�����м�������Ӷ����²ⶨ���_____ (����ƫ��������ƫ С��)��Ϊ֤�����������ȷ�������ʵ�鷽��������
ʵ�鷽�� | ���������� |
��ȡ��ƿ�еķ�ӦҺ�������� MnO2 ��ĩ | �д��������ݳ� |
���� NaOH ϡ��Һ�м��� 23 �η�̪��Һ,Ȼ����������ķ�ӦҺ | ��Һ�ȱ�����ɫ��ʼ���������� |
������ӦҺ�м��� 23 �η�̪��Һ,�����,Ȼ����μ��� ������ NaOH ϡ��Һ | �� NaOH ��Һ�� ������ɫ |
������ʵ����,�ܹ�֤�����������ȷ����ѷ�����_________ (��ʵ�����)��_______________��ʵ��ò���������ԭ����_____��
(3)������������ṩ���й���Ϣ�����һ��������ȷ�IJⶨ��Ʒ�Ĵ����������ʵ��������� Ҫ�ⶨ���й�����__________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1molE���������������ã��ڱ�״�����ܲ���33.6L H2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ��
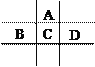
�ش��������⣺
��1��A��E�γɵĻ�����Ļ�ѧʽ�� __________________��
��2��B����������ﻯѧʽΪ____________��C��Ԫ������Ϊ ___________________ ��
��3��D�ĵ�����ˮ��Ӧ�ķ���ʽΪ____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���10.0mL 0.10 mol��L��1ij��Ԫ��H2R��Һ�еμ���ͬ���ʵ���Ũ�ȵ�NaOH��Һ�������Һ��pH��NaOH��Һ����ı仯����ͼ��ʾ������˵������ȷ����
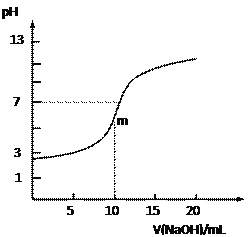
A. ���ж�H2R��ǿ�ỹ������
B. ������m��ʱ��Һ��c(Na+)>c(HR��) >c(R2��)>c(H+)
C. HR���ĵ�����������ˮ������
D. ��Һ��c(Na+)+c(H+)=c(HR��)+c(R2��)+c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һֱ��Ϊ���ĺ�������ڣ���1971��������ѧ�ҽ�F2ͨ��ϸ��ĭ���HFO(�η���)��
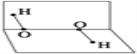
��1��HFO�ĵ���ʽΪ________��
��2��HFO��ˮ��Ӧ�õ�����A(�ṹ��ͼ��ʾ)��д��HFO��ˮ��Ӧ�Ļ�ѧ����ʽ________________��
��3������A�д���________����A���������� B���������� C��ͬʱ�������������� D��ͬʱ���ڼ��Լ��ͷǼ��Լ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ��
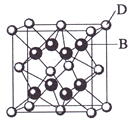
��ش�
(1)AԪ�ص������� ��
(2)B��Ԫ�ط����� ��C��Ԫ�ط����� ��B��A�γɵĻ������C ��A�γɵĻ�����е�ߣ���ԭ����
(3)E��Ԫ�����ڱ��е� ���ڣ��� ���Ԫ�أ���Ԫ�������� �� ����+2�����ӵĵ����Ų�ʽΪ ��
(4)��ͼ�п��Կ�����D��B�γɵ����ӻ�����Ļ�ѧʽΪ �������ӻ��� �ᄃ����ܶ�Ϊag��cm-3����������� (ֻҪ���г���ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮCoCl2Ϊ����ɫ����ˮ���Ϊ�ۺ�ɫ��ˮ������ˮ�������Ⱥ��ֱ����ˮCoCl2���ʳ���ʵ������������ʪ���Ϳ���ʪ��ָʾ����
CoCl2��xH2O![]() CoCl2��xH2O
CoCl2��xH2O
����ɫ�������������ۺ�ɫ
����65 g��ˮCoCl2����ˮ����CoCl2��xH2O 119 g��
��1��ˮ������x��________��
��2�����û�������Co2����λ��Ϊ6�����Ҿ������ⶨ��֪�ڽ�����ռ��Cl���ĸ�����Ϊ1��1�����仯ѧʽ�ɱ�ʾΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������¹�̷��¼�������������������Ƕ�ʳƷ�����Ŀֻţ���¹�̷��б������˱���Ϊ���������Ĺ�ҵԭ�������谷����֪�����谷�Ľṹ��ʽ��ͼ��ʾ�������谷���谷(H2N��C��N)�������壬��ش��������⣺
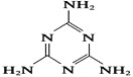
��1��д����̬̼ԭ�ӵĵ����Ų�ʽ______________��
��2���谷����C��N�еĵ�ԭ�ӡ������谷��״�ṹ�еĵ�ԭ�ӺͰ����еĵ�ԭ�ӣ������ֵ�ԭ�ӵ��ӻ�������ͷֱ���__________��__________��__________��
��3��һ�������谷��������______��������
��4�������谷������������![]() �������֮��ͨ�������ϣ������������γɽ�ʯ���������������Cԭ�Ӳ�ȡ________�ӻ����÷��ӵĽṹ��ʽ�У�ÿ��̼��ԭ��֮��Ĺ��ۼ���__________(��ѡ��)��
�������֮��ͨ�������ϣ������������γɽ�ʯ���������������Cԭ�Ӳ�ȡ________�ӻ����÷��ӵĽṹ��ʽ�У�ÿ��̼��ԭ��֮��Ĺ��ۼ���__________(��ѡ��)��
A��2������ B��2������ C��1��������1������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڷ�Ӧ:xR2++Cl2=yR3++zCl-��˵������ȷ���ǣ� ��
A.x=y,R2+�õ�����
B.x=2, Cl2��������
C.y=z,R3+�ǻ�ԭ����
D.x=z, Cl-����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com