
ЁОЬтФПЁПМзУб(CH3OCH3)ЪЧвЛжжаТаЭФмдДЁЃ
вбжЊЂйCO(g)+2H2(g)CH3OH(g) H1=-99kJ/mol
Ђк2CH3OH(g)CH3OCH3(g)+H2O(g) H2=-24kJ/mol
ЂлCO(g)+H2O(g)CO2(g)+H2(g) H3=-41kJ/mol
ЛиД№ЯТСаЮЪЬтЃК
(1)аДГіCOКЭH2ЗДгІЩњГЩCO2КЭCH3OCH3(g)ЕФШШЛЏбЇЗНГЬЪНЃК________________ЁЃ
(2)ЯТСаДыЪЉФмЬсИпЗДгІЂйжаCOЕФЦНКтзЊЛЏТЪЕФЪЧ________(ЬюзжФИ)ЁЃ
A.діДѓбЙЧП B.Щ§ИпЮТЖШ C.діДѓH2ХЈЖШ D.МгИпаЇДпЛЏМС
(3)дкКуЮТКуШнЬѕМўЯТжЛЗЂЩњЗДгІЂлЃЌЯТСаЧщПіБэУїИУЗДгІДяЕНЦНКтЕФЪЧ________(ЬюзжФИ)ЁЃ
A.ЦјЬхбЙЧПБЃГжВЛБф B.ЦјЬхУмЖШБЃГжВЛБф
C.![]() БЃГжВЛБф D.ХЈЖШЩЬБЃГжВЛБф
БЃГжВЛБф D.ХЈЖШЩЬБЃГжВЛБф
(4)дквЛЖЈЮТЖШ(TЁц)ЯТЃЌЯђКуШнУмБеШнЦїжаЭЖШывЛЖЈСПCH3OHЦјЬхЃЌжЛЗЂЩњЗДгІЂкЁЃЦјЬхЛьКЯЮяжаCH3OCH3ЕФЮяжЪЕФСПЗжЪ§[Іе(CH3OCH3)]гыЗДгІЪБМф(t)ЕФгаЙиЪ§ОнШчБэЫљЪОЁЃ
t/min | 0 | 15 | 30 | 45 | 80 | 100 |
[Іе(CH3OCH3)] | 0 | 0.05 | 0.08 | 0.09 | 0.10 | 0.10 |
Ђй30minЪБЃЌCH3OHЕФзЊЛЏТЪІС(CH3OH)________ЃЅЃЛИУЮТЖШЯТЃЌЩЯЪіЗДгІЕФЦНКтГЃЪ§K=________ЃЈгУЗжЪ§БэЪОЃЉЁЃ
ЂкЗДгІЫйТЪv=vе§-vФцЃЌЦфжаvе§=kе§Іе2(CH3OH)ЁЂvФц=kФцІе(CH3OCH3)Іе(H2O)ЃЌkе§ЁЂkФцЗжБ№ЮЊе§ЁЂФцЗДгІЫйТЪГЃЪ§ЃЌжЛгыЮТЖШгаЙиЃЌІеЮЊЮяжЪЕФСПЗжЪ§ЁЃ15minЪБ![]() ________(НсЙћБЃСє2ЮЛаЁЪ§)
________(НсЙћБЃСє2ЮЛаЁЪ§)
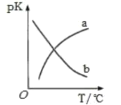
(5)дкУмБеШнЦїжаЗЂЩњЗДгІЂлЃЌЦНКтГЃЪ§ЮЊKЃЌpK=-lg2KЁЃpKЮТЖШЕФЙиЯЕШчЭМЫљЪОЃЌЭМжаЧњЯп________ЃЈЬюЁАaЁБЛђЁАbЁБЃЉФмЗДгГЦНКтГЃЪ§БфЛЏЧїЪЦЁЃ
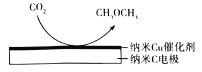
(6)дкCO2ДІРэЙ§ЕФБЅКЭKHCO3ШмвКжаЃЌЕчНтЛюЛЏЕФCO2жЦБИCH3OCH3ЕФдРэШчЭМЫљЪОЃЌвѕМЋгаHCO3-ЩњГЩЃЌИУЕчЕФЕчМЋЗДгІЪНЮЊ________ЁЃ
ЁОД№АИЁП3CO(g)+3H2(g)CO2(g)+CH3OCH3(g) H=-263kJ/mol AB CD 16 ![]() 5.06 a 14CO2+12e-+9H2O=CH3COH3+12HCO3-
5.06 a 14CO2+12e-+9H2O=CH3COH3+12HCO3-
ЁОНтЮіЁП
(1)ИљОнИЧЫЙЖЈТЩ2Ђй+Ђк+ЂлПЩЕУЂмЃЛ
(2)ИљОнРеЯФЬиСадРэХаЖЯЁЃ
(3)ЗДгІЂлжаЦјЬхЕФМЦСПЪ§зѓгвСНБпЯрЕШЃЌдђЗДгІздЪМжСжеЃЌШнЦїЕФбЙЧПЁЂЮяжЪЕФСПЁЂУмЖШВЛБфЃЌВЛФмзїЮЊЦНКтЕФБъжОЃЌЗДгІЦНКтЪБЃЌn(CO)ЁЂn(CO2)ЕФСПВЛдйИФБфЃЌХЈЖШВЛдйИФБфЃЛ
(4)ИљОнБэжаЪ§ОнЃЌЗДгІдй80minЪБДяЕНЦНКтзДЬЌЃЌІе(CH3OCH3)ЮЊ0.10ЃЌ
(5)ЗДгІЂлЕФьЪБфаЁгкСуЃЌЮЊЗХШШЗДгІЃЌЩ§ИпЮТЖШЦНКтФцЯђвЦЖЏЃЌЦНКтГЃЪ§МѕаЁЃЛ
(6)ЕчНтвѕМЋЕУЕчзггЩCO2ЩњГЩCH3OCH3ЪБЃЌгаHCO3-ЩњГЩЁЃ
(1)Ђм3CO(g)+3H2(g)CO2(g)+CH3OCH3(g)ЃЌИљОнИЧЫЙЖЈТЩ2Ђй+Ђк+ЂлПЩЕУЂмЃЌ3CO(g)+3H2(g)CO2(g)+CH3OCH3(g) H=-263kJ/molЃЛ
(2)ЬсИпЗДгІЂйжаCOЕФЦНКтзЊЛЏТЪЃЌдђЦНКте§ЯђвЦЖЏЃЌИљОнРеЯФЬиСадРэдіДѓбЙЧПКЭНЕЕЭЮТЖШПЩЪЙЦНКте§ЯђвЦЖЏЛђЬсИпЧтЦјЕФгУСПЃЌД№АИЮЊABЃЛ
(3)ЗДгІЂлжаЦјЬхЕФМЦСПЪ§зѓгвСНБпЯрЕШЃЌдђЗДгІздЪМжСжеЃЌШнЦїЕФбЙЧПЁЂЮяжЪЕФСПЁЂУмЖШВЛБфЃЌВЛФмзїЮЊЦНКтЕФБъжОЃЌЗДгІЦНКтЪБЃЌn(CO)ЁЂn(CO2)ЕФСПВЛдйИФБфЃЌХЈЖШВЛдйИФБфЃЌД№АИЮЊCDЁЃ
(4)ИљОнБэжаЪ§ОнЃЌЗДгІдй80minЪБДяЕНЦНКтзДЬЌЃЌІе(CH3OCH3)ЮЊ0.10ЃЌ
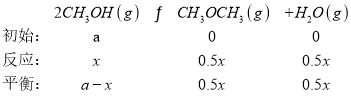
Ђй30minЪБЃЌИљОнШ§ЖЮЪНЃЌ![]() =0.08ЃЌНтЕФx=0.16aЃЌCH3OHЕФзЊЛЏТЪІС(CH3OH)=
=0.08ЃЌНтЕФx=0.16aЃЌCH3OHЕФзЊЛЏТЪІС(CH3OH)=![]() ЁС100%=16%ЃЛЭЌРэЃЌЦНКтЪБx=0.2ЃЌK=
ЁС100%=16%ЃЛЭЌРэЃЌЦНКтЪБx=0.2ЃЌK=![]() =
=![]() ЃЛ
ЃЛ
Ђк15minЪБЃЌИљОнШ§ЖЮЪНЃЌ![]() =0.05ЃЌНтЕФx=0.1aЃЌдђІе(CH3OCH3)=Іе(H2O)=0.05aЃЌІе2(CH3OH)=(0.9a)2ЃЌЦНКтЪБЃЌvе§=kе§Іе2(CH3OH)=vФц=kФцІе(CH3OCH3)Іе(H2O)ЃЌ
=0.05ЃЌНтЕФx=0.1aЃЌдђІе(CH3OCH3)=Іе(H2O)=0.05aЃЌІе2(CH3OH)=(0.9a)2ЃЌЦНКтЪБЃЌvе§=kе§Іе2(CH3OH)=vФц=kФцІе(CH3OCH3)Іе(H2O)ЃЌ![]() =KЃЌ
=KЃЌ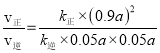 =
=![]() =5.06ЃЛ
=5.06ЃЛ
(5)ЗДгІЂлЕФьЪБфаЁгкСуЃЌЮЊЗХШШЗДгІЃЌЩ§ИпЮТЖШЦНКтФцЯђвЦЖЏЃЌЦНКтГЃЪ§МѕаЁЃЌpK=-lg2KЃЌдђpKдіДѓЃЌaФмЗДгГЦНКтГЃЪ§БфЛЏЧїЪЦЃЛ
(6)дкCO2ДІРэЙ§ЕФБЅКЭKHCO3ШмвКжаЃЌЕчНтвѕМЋЕУЕчзгЃЌгЩCO2ЩњГЩCH3OCH3ЪБЃЌгаHCO3-ЩњГЩЃЌЕчМЋЗДгІЪНЮЊ14CO2+12e-+9H2O=CH3COH3+12HCO3-ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЩшNAЮЊАЂЗќМгЕТТоГЃЪ§ЕФжЕЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
A.60gЖўбѕЛЏЙшОЇЬхжаКЌга2NAИіЙшбѕМќB.1molD2OжаКЌга10NAИіжЪзг
C.12gН№ИеЪЏжаКЌгаNAИіЬМЬММќD.1molЪЏФЋОЇЬхжаКЌга2NAИіЬМЬММќ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдЫЫЭЁАЩёжлЁБЮхКХЗЩДЌЕФЛ№М§ШМСЯГ§вКЬЌЫЋбѕЫЎЭтЃЌЛЙгаСэвЛжжвКЬЌЕЊЧтЛЏКЯЮяЁЃвбжЊИУЛЏКЯЮяжаЧтдЊЫиЕФжЪСПЗжЪ§ЮЊ12.5ЃЅЃЌЯрЖдЗжзгжЪСПЮЊ32ЃЌНсЙЙЗжЮіЗЂЯжИУЗжзгНсЙЙжажЛгаЕЅМќЁЃ
ЃЈ1ЃЉИУЕЊЧтЛЏКЯЮяЕФЕчзгЪНЮЊ_________ЁЃ
ЃЈ2ЃЉИУЮяжЪгывКЬЌЫЋбѕЫЎЗДгІФмВњЩњСНжжЮоЖОгжВЛЮлШОЛЗОГЕФЮяжЪЃЌаДГіИУЗДгІЕФЛЏбЇЗНГЬЪН_______________________ЁЃ
ЃЈ3ЃЉNH3ЗжзгжаЕФNдзггавЛЖдЙТЖдЕчзг ФмЗЂЩњЗДгІЃКNH3+HCl=NH4ClЁЃЪдаДГіЩЯЪіЕЊЧтЛЏКЯЮяЭЈШызуСПбЮЫсЪБЃЌЗЂЩњЗДгІЕФЗНГЬЪНЁЃ
ФмЗЂЩњЗДгІЃКNH3+HCl=NH4ClЁЃЪдаДГіЩЯЪіЕЊЧтЛЏКЯЮяЭЈШызуСПбЮЫсЪБЃЌЗЂЩњЗДгІЕФЗНГЬЪНЁЃ
_________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЛЏКЯЮяHЪЧвЛжжгаЛњЙтЕчВФСЯжаМфЬхЁЃЪЕбщЪвгЩЗМЯуЛЏКЯЮяAжЦБИHЕФвЛжжКЯГЩТЗЯпШчЯТЃК

вбжЊЃКЂйRCHO+CH3CHO ![]() RCH=CHCHO+H2OЃЛЂк
RCH=CHCHO+H2OЃЛЂк![]() ЁЃ
ЁЃ
ЛиД№ЯТСаЮЪЬтЃК
(1)CЕФНсЙЙМђЪНЮЊ_______________________ЁЃ
(2)FжаЙйФмЭХЕФУћГЦЮЊ___________________ЁЃ
(3)BгыаТжЦCu(OH)2аќзЧвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ________________ЁЃ
(4)ДгзЊЛЏСїГЬЭМПЩМћЃЌгЩDЕНEЗжСНВННјааЃЌЦфжаЕквЛВНЗДгІЕФРраЭЮЊ__________ЁЃ
(5)ЛЏКЯЮяGЕФЖўТШДњЮяга__________жжЭЌЗжвьЙЙЬхЁЃ
(6)ЗМЯуЛЏКЯЮяXЪЧDЕФЭЌЗжвьЙЙЬхЃЌXФмЗЂЩњвјОЕЗДгІЃЌЦфКЫДХЙВеёЧтЦзЯдЪОга3жжЛЏбЇЛЗОГЕФЧтЃЌЗхУцЛ§жЎБШЮЊ6ЁУ1ЁУ1ЃЌЗћКЯЬѕМўЕФXЕФНсЙЙЙВгаЖржжЃЌШЮаДГі2жжЗћКЯвЊЧѓЕФXЕФНсЙЙМђЪН________ЁЂ___________ЁЃ
(7)аДГігУМзШЉКЭввШЉЮЊдСЯжЦБИЛЏКЯЮяCH2=CHCOOCH3ЕФКЯГЩТЗЯпЃЈЦфЫћЪдМСШЮбЁЃЉ____ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП(1)АДЯЕЭГУќУћЗЈУќУћЃК
гаЛњЮяCH3CH(C2H5)CH(CH3)2ЕФУћГЦЪЧ________________________ЁЃ
(2)аДГіЯТСаИїжжгаЛњЮяЕФНсЙЙМђЪНЃК
Ђй2,3ЖўМзЛљ4ввЛљМКЭщ_________________ЃЛ
ЂкжЇСДжЛгавЛИіввЛљЧвЯрЖдЗжзгжЪСПзюаЁЕФЭщЬў___________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП![]() ЮЊАЂЗќМгЕТТоГЃЪ§ЕФжЕЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
ЮЊАЂЗќМгЕТТоГЃЪ§ЕФжЕЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
A. 0.1 mol ЕФ![]() жаЃЌКЌга
жаЃЌКЌга![]() Иіжазг
Иіжазг
B. pH=1ЕФH3PO4ШмвКжаЃЌКЌга![]() Иі
Иі![]()
C. 2.24LЃЈБъзМзДПіЃЉБНдкO2жаЭъШЋШМЩеЃЌЕУЕН![]() ИіCO2Зжзг
ИіCO2Зжзг
D. УмБеШнЦїжа1 mol PCl3гы1 mol Cl2ЗДгІжЦБИ PCl5ЃЈgЃЉЃЌдіМг![]() ИіP-ClМќ
ИіP-ClМќ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖЬжмЦкжїзхдЊЫи XЁЂYЁЂZЁЂW дзгађЪ§вРДЮдіДѓЃЌX ЪЧЕиПЧжаКЌСПзюЖрЕФдЊЫиЃЌY дзгЕФзюЭтВужЛгавЛИіЕчзгЃЌZ ЮЛгкдЊЫижмЦкБэЂѓAзхЃЌW гыXЪєгкЭЌвЛжїзхЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
A. дзгАыОЖЃКr(W) > r(Z) > r(Y)
B. гЩXЁЂY зщГЩЕФЛЏКЯЮяжаОљВЛКЌЙВМлМќ
C. Y ЕФзюИпМлбѕЛЏЮяЕФЫЎЛЏЮяЕФМюадБШZЕФШѕ
D. X ЕФМђЕЅЦјЬЌЧтЛЏЮяЕФШШЮШЖЈадБШWЕФЧП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЗДгІЕФгаЛњВњЮяжаЃЌгаЕФгаЭЌЗжвьЙЙЬхЃЌгаЕФУЛгаЭЌЗжвьЙЙЬхЃЌЦфжавЛЖЈВЛДцдкЭЌЗжвьЙЙЬхЕФЗДгІЪЧ
A.вьЮьЖўЯЉ(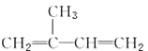 )гыЕШЮяжЪЕФСПЕФBr2ЗЂЩњМгГЩЗДгІ
)гыЕШЮяжЪЕФСПЕФBr2ЗЂЩњМгГЩЗДгІ
B.2ЃТШЖЁЭщгыNaOHввДМШмвКЙВШШЗЂЩњЯћШЅHClЗжзгЕФЗДгІ
C.МзБНдквЛЖЈЬѕМўЯТЗЂЩњЯѕЛЏЗДгІЩњГЩвЛЯѕЛљМзБНЕФЗДгІ
D.БНЗггыNa2CO3ШмвКЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП1LФГШмвКжаКЌгаЕФРызгШчЯТБэЃК
Рызг |
|
|
|
|
ЮяжЪЕФСПХЈЖШ
| 1 | 1 |
| 1 |
гУЖшадЕчМЋЕчНтИУШмвКЃЌЕБЕчТЗжага![]() ЭЈЙ§ЪБЃЈКіТдЕчНтЪБШмвКЬхЛ§ЕФБфЛЏМАЕчМЋВњЮяПЩФмДцдкЕФШмНтЯжЯѓЃЉЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ
ЭЈЙ§ЪБЃЈКіТдЕчНтЪБШмвКЬхЛ§ЕФБфЛЏМАЕчМЋВњЮяПЩФмДцдкЕФШмНтЯжЯѓЃЉЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ
A.ЕчНтКѓЛьКЯШмвКЕФpH=0B.a=3
C.бєМЋЩњГЩ![]() D.вѕМЋЮіГіЕФН№ЪєЪЧЭгыТС
D.вѕМЋЮіГіЕФН№ЪєЪЧЭгыТС
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com