
�ѣ�Ti������Ϊ��������֮��ĵ����������Ѱף�TiO2����Ŀǰ��õİ�ɫ���ϡ��Ʊ�TiO2��Ti��ԭ�����������ҹ�������������������λ������Fe2O3����������Ҫ�ɷ�ΪFeTiO3����ȡTiO2���������£�
�� Ti��ԭ������Ϊ22��Tiλ��Ԫ�����ڱ��е�_____���ڣ���______�塣
�Ʋ���ټ�����Ŀ����_________________________________________________��
�������ȴ��Ŀ����_________________________________________________��
�� �����Ʊ�TiO2�Ĺ����У��������õĸ�������___________�����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����_________________������
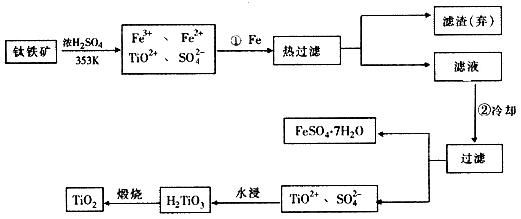
�� �ɽ��ʯ(TiO2)��ȡ����Ti���漰���IJ���Ϊ��
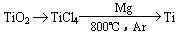
��֪��
�� C(s) + O2(g) == CO2(g)�� ��H = ��393.5 KJ��mol-1
�� 2CO(g) + O2(g) == 2CO2(g)�� ��H =��566 KJ��mol-1
�� TiO2(s) + 2Cl2(g) == TiCl4(s) + O2(g)�� ��H = +141 KJ��mol-1
��TiO2(s) + 2Cl2(g) + 2C(s)== TiCl4(s) + 2CO(g) �ġ�H = ____________��
��ӦTiCl4 + 2Mg == 2MgCl2 + Ti ��Ar�����н��е�������___________________��
���𰸡�
��1��4 IVB
��2����Fe3+��ԭΪFe2+ ����������롢��õ� ��FeSO4��7H2O
��FeSO4��7H2O
��3��FeSO4��7H2O ʯ�ң���̼��ơ��ϼ
��4��-80 kJ��mol-1
��ֹ������Mg(Ti)������е�O2����CO2��N2������
��������������һ�����͵������ͼ�Ʊ��⡣��������Ի���֪ʶ�����գ�����Ҫ����ѧ��֪ʶ���ۺ�Ӧ��������
��1����ԭ�������ƶ�Ԫ�������ڱ��е�λ�ã��ؼ�����ϤԪ�����ڱ��Ľṹ������������ֹԪ�ص�ԭ����������������λ�õȡ�Ti��ԭ������Ϊ22������Ϥ��Ca��ԭ������Ϊ20���������Ca��Ԫ�����ڱ��е�λ�ã������ƶ�Tiλ�ڵ������ڣ��ڢ�B�塣
��2���ɿ�ͼ��ʾ���̷�����֪��Ҫ��TiO2+��Fe3+��Fe2+���룬��Ҫ�ȼ������۽�Fe3+��ԭΪFe2+�����ȹ��˳�ȥ�������ٽ���Һ��ȴʹFeSO4��7H2O������
��3���Ʊ�TiO2�����еĸ�����FeSO4��7H2O�ڹ�ũҵ�������ճ��������н϶��Ӧ�á��ڷ����H2TiO3�ķ�Һ�У�������������TiO2+��Fe2+���ɼ���ʯ�ң���̼��ơ��ϼ����H+��Ũ�ȣ�ʹ֮ת��Ϊ��������������ѭ�����á�
��4����Ӧ��ֻ�뷴Ӧ��ʼ̬����̬�йأ��������ķ�Ӧ���е�;���ء�����֪�Ȼ�ѧ����ʽ��Ӽ���+2���٣��ڵ�TiO2��s��+2Cl2��g��+2C��s��==TiCl4��s��+2CO��g�����䦤H=141 kJ��mol��1+2������393.5 kJ��mol��1��������566 kJ��mol��1��=��80 kJ��mol��1����Ϊ�ڸ����£�Mg��Ti��������е�O2����CO2��N2�����ã�������Mg��ԭTiCl4ʱҪ�ڶ������գ�Ar���н��С�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������AgNO3��BaCl2��K2SO3��Mg(NO3)2������Һ�����������Ǽ�����Ӧ�����Լ�����
A. ���ᡢ���� B. ���ᡢ����������Һ
C. ��ˮ������ D. ��ˮ������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�˱�����ͭ������ͭ�̣����·�����ȷ����
A ����ͭ����������������
B ����ͭ�������ڸ���Ļ�����
C ����ͭ�������ڳ�ʪ�Ŀ�����
D ����ͭ���ı��渲��һ������ĸ߷���Ĥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ���£�
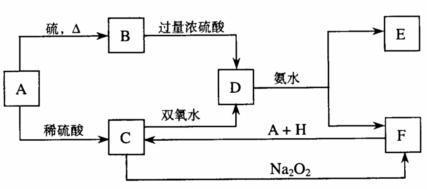
��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫ���塣��ش��������⣺
(1)���ɫ������F����ֱ����С�ķ�Χ��________________��
(2)A��B��H�Ļ�ѧʽ��A________��B________��H________��
(3)��H2O2���ӵĵ���ʽ��________________��
��д��C��������Һ��˫��ˮ��Ӧ�����ӷ���ʽ��
_____________________________________________________________________��
(4)д������E�������ӵ�ʵ�鷽��������__________________________________________
_____________________________________________________________________��
(5)��C��Һ�м�����C�����ʵ�����Na2O2��ǡ��ʹCת��ΪF��д���÷�Ӧ�����ӷ���ʽ��
_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʹ0.1mol/L��NaHCO3��Һ��c(H+)��c(CO32��)��c(HCO3��)�����٣��䷽����
A��ͨ�������̼���� B�������������ƹ���
C��ͨ���Ȼ������� D�����뱥��ʯ��ˮ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�顣
��1����ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ����壨��״������ͬ�����Ϊb L��
��Ӧ�Ļ�ѧ����ʽ��__________________________________����Ʒ������������_______________g��
��2����ȡa g��Ʒ�����ȼ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�
_________________________����������������������_____________��
��3������2���з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L���������루1������������������c��b��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ԭ��Ӧ�У�ˮ��Ϊ���������ǣ� ����
A��C+H2O CO+H2 B��3NO2+H2O==2HNO3+NO
CO+H2 B��3NO2+H2O==2HNO3+NO
C��2Na2O2+2H2O==4NaOH+O2�� D��2F2+2H2O==4HF+O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڸ�����ұ���У�����CO����ͨ����ȵ��������У������ķ�Ӧ�ǣ�Fe2O3+3CO 2Fe+3CO2������գ�
2Fe+3CO2������գ�
��1����������Ӧ�У�________Ԫ�صĻ��ϼ����ߣ����Ԫ�ص�ԭ��________���ӣ�����������Ӧ����________Ԫ�صĻ��ϼ۽��ͣ����Ԫ�ص�ԭ��________���ӣ�����________��Ӧ��
��2���÷�Ӧ��________����������������________��Ӧ��________�ǻ�ԭ����������________��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�˵����ȷ���ǣ� ��
A����0.2mol/L��NH3��H2O��0.1mol/L��HCl��Һ�������Ϻ�PH >7��
��c(Cl-)��c(NH4+)��c(OH-)��c(H+)
B����֪MgCO3��Ksp=6.82��10-6�������к��й���MgCO3����Һ�У�����
C��Mg2+��=C��CO32-�� ����C��Mg2+����C��CO32-��==6.82��10-6 mol•L��1
C�� 0.1mol/LNa2CO3��0.1mol/LNaHCO3��Һ�������ϣ�
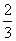 c(Na��)��c(CO32��)��c(HCO3��)��c(H2CO3)
c(Na��)��c(CO32��)��c(HCO3��)��c(H2CO3)
D���ö��Ե缫���Na2SO4��Һ������������������ʵ���֮��Ϊ2:1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com