
Ϊȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�顣
��1����ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ����壨��״������ͬ�����Ϊb L��
��Ӧ�Ļ�ѧ����ʽ��__________________________________����Ʒ������������_______________g��
��2����ȡa g��Ʒ�����ȼ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�
_________________________����������������������_____________��
��3������2���з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L���������루1������������������c��b��________________��
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ҷ�ͪ������ҽҩ�м��弰�����߹̻��������ɶ���������ͪ�����Ƶã���Ӧ�Ļ�ѧ����ʽ��װ��ͼ(����װ��ʡ��)���£�
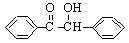 ��2FeCl3
��2FeCl3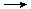
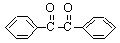 ��2FeCl2��2HCl
��2FeCl2��2HCl
�ڷ�Ӧװ���У�����ԭ�ϼ��ܼ��������¼��Ȼ�������Ӧ�����������У���ȴ���ж������Ҷ�ͪ�ֲ�Ʒ��������70���Ҵ�ˮ��Һ�ؽᾧ�ᴿ���ؽᾧ���̣�
�����ܽ������̿��ɫ�����ȹ��ˡ���ȴ�ᾧ�����ˡ�ϴ�ӡ�����
��ش��������⣺
��1��д��װ��ͼ�в������������ƣ�a___________��b____________��
��2�����ȹ��˺���Һ��ȴ�ᾧ��һ������£�������Щ���������ڵõ��ϴ�ľ��壺____��
A��������ȴ��Һ B����ҺŨ�Ƚϸ�
C�������ܽ�Ƚ�С D�����������ܼ�
�����Һ�з������������ɲ���__________�ȷ����ٽ�����������
��3���������õ���ֽӦ��_______������ڡ���С�ڡ�������©���ھ�����ȫ��С��ס���ձ��еĶ������Ҷ�ͪ����ת�벼��©��ʱ��������������ճ���������壬��ѡ��Һ�彫�����ϵľ����ϴ������ת�벼��©��������Һ������ʵ���________��
A����ˮ�Ҵ� B������NaCl��Һ C��70���Ҵ�ˮ��Һ D����Һ
��4�������ؽᾧ�����е���һ��������ȥ�˲��������ʣ�___________��
��5��ijͬѧ���ñ���ɫ��(ԭ���Ͳ�����ֽ������ͬ)���ٷ�Ӧ���̣��ֱ��ڷ�Ӧ��ʼ������15min��30min��45min��60minʱ����ëϸ��ȡ��������������ɫ��չ����İߵ���ͼ��ʾ����ʵ�������±ȽϺ��ʵĻ���ʱ����________��
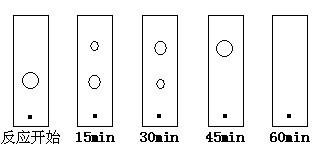
A��15min B��30min C��45min D��60min
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ������A��M��һ�������µ�ת����ϵ�����ֲ��P��Ӧ����δ�г��������У�I���ɵ�������Ԫ����ɵĵ������۵���ߵĽ�����K��һ�ֺ���ɫ���塣
����д���пհף�
��1�������ڱ��У���ɵ���G��Ԫ��λ�ڵ�_______���ڵ�_______�塣
��2���ڷ�Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ___________________��
��3���ڷ�Ӧ�ڡ��ۡ��ޡ����У������ڻ��Ϸ�Ӧ�����ڷ�������ԭ��Ӧ����_________
����д��ţ���
��4����Ӧ�� �����ӷ���ʽ�ǣ�_______________________________________
��5����������D ��KNO3��KOH ���ڣ����Ƶ�һ�֡���ɫ��������Ч��ˮ��K2FeO4 ��������أ�.ͬʱ������KNO2��H2O ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�
_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л��ۺ�����������ȼ���������Ӿۺ����ʹ�ð�ȫ�ԣ�������Ӧ�÷�Χ�����磬��ij����ϩ��֬�м����������������Ʊ�����ȼ��Mg(OH)2����֬��ȼ�Դ�͡���Mg(OH)2�������������£�

�ž���±ˮ�е�MgCl2������ʯ���鷴Ӧ�ϳɼ�ʽ�Ȼ�þ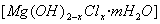 ����Ӧ�Ļ�ѧ����ʽΪ ��
����Ӧ�Ļ�ѧ����ʽΪ ��
�ƺϳɷ�Ӧ������393 K��523 K��ˮ�ȴ���8 h��������Ӧ��
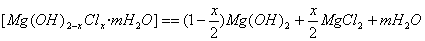
ˮ�ȴ������ˡ�ˮϴ��ˮϴ��Ŀ���� ��
����ȼ��Mg(OH)2���о�������ɢ����߷��Ӳ��������Ժõ��ص㡣������������������йصIJ����� ��
����֪�Ȼ�ѧ����ʽ��
Mg(OH)2(s)==MgO(s)+H2O(g)�� ��H1=+81.5 kJ��mol-1
Al(OH)3(s)=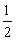 Al2O3(s)+
Al2O3(s)+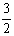 H2O(g)�� ��H2=+87.7 kJ��mol-1
H2O(g)�� ��H2=+87.7 kJ��mol-1
��Mg(OH)2��Al(OH)3����ȼ���õ���Ҫԭ���� ��
�ڵ�����Mg(OH)2��Al(OH)3��ȣ���ȼЧ���Ϻõ��� ��
ԭ���� ��
�ɳ�����ȼ����Ҫ�� ���ࣺA.±ϵ�����������飻B.��ϵ����������������C.���࣬��Ҫ��Mg(OH)2��Al(OH)3���ӻ����ĽǶȿ��ǣ�Ӧ��ʱ���������ȼ���� (�����)�������� ��
���ࣺA.±ϵ�����������飻B.��ϵ����������������C.���࣬��Ҫ��Mg(OH)2��Al(OH)3���ӻ����ĽǶȿ��ǣ�Ӧ��ʱ���������ȼ���� (�����)�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ѣ�Ti������Ϊ��������֮��ĵ����������Ѱף�TiO2����Ŀǰ��õİ�ɫ���ϡ��Ʊ�TiO2��Ti��ԭ�����������ҹ�������������������λ������Fe2O3����������Ҫ�ɷ�ΪFeTiO3����ȡTiO2���������£�
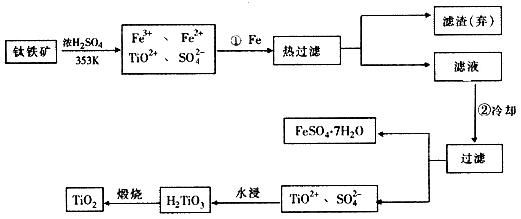
�� Ti��ԭ������Ϊ22��Tiλ��Ԫ�����ڱ��е�_____���ڣ���______�塣
�Ʋ���ټ�����Ŀ����_________________________________________________��
�������ȴ��Ŀ����_________________________________________________��
�� �����Ʊ�TiO2�Ĺ����У��������õĸ�������___________�����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����_________________������
�� �ɽ��ʯ(TiO2)��ȡ����Ti���漰���IJ���Ϊ��
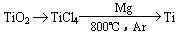
��֪��
�� C(s) + O2(g) == CO2(g)�� ��H = ��393.5 KJ��mol-1
�� 2CO(g) + O2(g) == 2CO2(g)�� ��H =��566 KJ��mol-1
�� TiO2(s) + 2Cl2(g) == TiCl4(s) + O2(g)�� ��H = +141 KJ��mol-1
��TiO2(s) + 2Cl2(g) + 2C(s)== TiCl4(s) + 2CO(g) �ġ�H = ____________��
��ӦTiCl4 + 2Mg == 2MgCl2 + Ti ��Ar�����н��е�������___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
19����ĩ���й���ʼ�˰����������ҵ�����������������۵ķ�չ���̣��й��ֲ�����20����ĩͻ��һ�ڶ֣��������һλ��
42.��1��������ͨ����¯ұ�����ã�ұ��������һ����̼��ԭ������Ļ�ѧ����ʽΪ ��
��2������һ�������¿�����ַǽ������ʷ�Ӧ������������Ӧ�Ļ�ѧ����ʽΪ ��������Ӧ�Ļ�ѧ����ʽΪ ��
43.����һ��м����м�Ļ����110g ,������м����������Ϊ49%���Լ���û����������ϡ���ᷴӦ���ͷų���������������״������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ԭ��Ӧ��һ����Ҫ�Ļ�ѧ��Ӧ���㷺��������Ȼ���У������ǵ���������������ʮ����Ҫ�����á�����Ϊ���ж�������ԭ��Ӧ����������ȷ���ǣ� ����
A���϶��е���ת��
B���϶����й���Ԫ�صķ�Ӧ
C���϶���Ԫ�ػ��ϼ۵ı仯
D���������õ����ӵ������뻹ԭ��ʧȥ���ӵ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ� ��
A���ҹ������з��ġ���о1�š�CPUоƬ����ά��ͬ�ֲ���
B����Ҫͨ����ѧ��Ӧ���ܴӺ�ˮ�л��ʳ�κ͵�ˮ
C��ˮ�������Ͳ����ϵĴ��̶��ǹ�������Ʒ
D���ֹ��Ʊ��������漰������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ķ�չ�봴������ֹ�����ҹ���ѧ�Һ�°�ĸ����Ĵ����������գ��������̿ɼ�Ҫ��ʾ��ͼK39��4��

ͼK39��4
(1)���������Ҫͨ��CO2�Ͱ�����Ӧѡͨ��______ (�ѧʽ)��ԭ����_________________________________________��
(2)�������з�����Ӧ�Ļ�ѧ����ʽ��__________________________________��
(3)ĸҺ�е�������Ҫ��________����ĸҺ��ͨ��������ϸСʳ�ο�������ȴ��������Ʒ��ͨ�백����������_____________________________________________��
(4)ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%���ϣ���Ҫ�������________(�����������еı��)��ѭ��������X��__________���ӳ���������ȡ�����IJ�����__________ ��
(5)д������¯�з�����Ӧ�Ļ�ѧ����ʽ_____________________________��
(6)�����ƵõIJ�Ʒ̼�����п��ܺ��е�������____________(�ѧʽ)��Ϊ������� �ʵĴ��ڣ����������__________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com