
�������������ǹذ��Լ�������˵��������Ϊ����ȷ���ǣ� ��
|
 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ������ѧ�ڵ�һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(��15��)
�л��������ұ�(GB2760��200��)�涨���Ѿ���SO2���ʹ����Ϊ0.25g��L��1.ij��ȤС����ͼIװ��(�г�װ����)�ռ�ij���Ѿ���SO2,�����京�����вⶨ.

 ��1������A�������� ��ˮͨ��A�Ľ���Ϊ ��
��1������A�������� ��ˮͨ��A�Ľ���Ϊ ��
��2��B�м���300.00mL���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����C��H2O2��ȫ��Ӧ���仯ѧ����ʽΪ ��
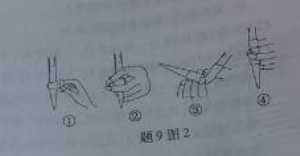
��3����ȥC�й�����H2O2��Ȼ����0.0900mol��L��1NaOH����Һ���еζ����ζ�ǰ������ʱ��Ӧѡ����9ͼ2�е� �����ζ��յ�ʱ��Һ��pH��8.8����ѡ���ָʾ��Ϊ ������50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶ȡ�10�����������Һ������ (�����)
(�٣�10mL���ڣ�40mL���ۣ�10mL���ܣ�40mL)
��4���ζ����յ�ʱ������NaOH��Һ25.00mL�������Ѿ���SO2����Ϊ g��L��1
��5���òⶨ�����ʵ��ֵƫ�ߣ�����ԭ����������װ������Ľ���ʩ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ������ѧ�ڵ�һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ˮ��Һ���ܴ��������һ��������
A��Na+��Ca2+��Cl-��SO42- B��Fe2+��H+��SO32-��ClO-
C��Mg2+��NH4+��Cl-��SO42- D��K+��Fe3+��NO3-��SCN-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ����������и߶���ѧ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪������C6H6��������B3N3H6�������ķ��ӽṹ���ƣ�����ͼ�����Ķ���ȡ����B3N3H4Cl2��ͬ���칹�����ĿΪ
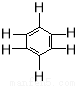

A. 6 B. 4 C. 3 D. 2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ����������и߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵�����ʾ������ȷ����
A�������������������������ֱ���ȫȼ�գ����߷ų�������
B���ɡ�C(ʯī)��C(���ʯ)��H = +1.9kJ/mol����֪�����ʯ��ʯī�ȶ�
C����ϡ��Һ�У���+(aq)+OH-(aq)��H2O(l) ��H = - 57.3kJ/mol��������1molCH3COOH�뺬1mol NaOH����Һ��ϣ��ų�������С��57.3kJ
D����101kPaʱ,2gH2��ȫȼ������Һ̬ˮ,�ų�285.8kJ����������ȼ�յ��Ȼ�ѧ����ʽ��Ϊ:2H2(g)+O2(g)��2H2O(l)��H = ��285.8kJ/mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com