
����Ŀ��NaClO2��һ��Ư�ס�������,�㷺Ӧ����ֽ�����ĵ�����Ư�ס�һ���Ʊ�NaClO2�ֲ�Ʒ�Ĺ�����������ͼ��ʾ��
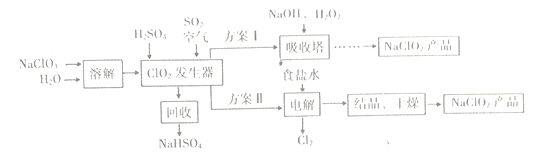
��֪:�ٴ�����ClO2���ֽ��������ը��
��NaClO2������Һ�ڵ���38��ʱ����NaClO2��3H2O����;����38��ʱ����
NaClO2����;����60��ʱNaClO2�ֽ�����NaClO3��NaCl��
�Իش��������⣺
��1���������й�����������,��Ŀ����____(ѡ�����)��
a.��SO2������SO3,����ǿ����
b.ϡ��CO2����,�Է�ֹ������ը
c.��������������ȫ������,�Լ���ClO2��ʧ
��2���������з�Ӧ�����ӷ���ʽΪ___
��3��ClO2�������з�Ӧ�Ļ�ѧ����ʽΪ___________
��4�����������пɻ�� NaClO2��Һ,��NaClO2��Һ��NaCO2��Ʒ,�����IJ�����������Ϊ:��______���¼�ѹ�����ᾧ;��________����ϴ��;�ܵ��¸���,�õ���Ʒ��
��5����������������ĵ缫��Ӧʽ��_________��������Ӧ����Ҫ������_________��
���𰸡� bc 2ClO2��H2O2��2OH�� = 2ClO2����O2��2H2O 2NaClO3��SO2��H2SO4 === 2ClO2��2NaHSO4 38�桫60�� ���� 2Cl����2e�� = Cl2�� ClO2������NaClO2��
����������1��ͨ�����̹��̿��Կ������������й���������������Ŀ����ϡ��ClO2�������Է�ֹ������ը����������������ȫ���������Լ���ClO2��ʧ�����������Ѿ����������ˣ������Ѿ���ǿ�����Խ�SO2������SO3������ǿ�����Ǵ���ģ���ȷѡ��bc��
��2��ClO2��H2O2�ڼ��Ի����£�����NaClO2��H2O2�ڸ÷�Ӧ������ԭ����������Ϊ��������Ӧ�����ӷ���ʽΪ2ClO2��H2O2��2OH�� = 2ClO2����O2��2H2O����ȷ����2ClO2��H2O2��2OH�� = 2ClO2����O2��2H2O��
��3��NaClO3����������������ԭΪClO2��SO2������Ϊ��������ӣ�����ClO2��ѧ����ʽΪ2NaClO3��SO2��H2SO4 === 2ClO2��2NaHSO4����ȷ����2NaClO3��SO2��H2SO4 === 2ClO2��2NaHSO4��
��4�����������Ϣ��NaClO2������Һ�ڵ���38��ʱ����NaClO2��3H2O����������38��ʱ������NaClO2�������60��ʱNaClO2�ֽ�����NaClO3��NaCl��֪�����������пɻ�� NaClO2��Һ����NaClO2��Һ��NaCO2��Ʒ�������IJ�����������Ϊ���ٿ���38����60���¼�ѹ�����ᾧ���ڹ�������ϴ�����ܵ��¸������õ���Ʒ����ȷ����38�桫60�� �� ������
��5����ⱥ��ʳ��ˮ���������������������ĵ缫��Ӧʽ��2Cl����2e�� = Cl2�� �� ClO2�������õ��ӻ�ԭΪClO2������NaClO2������ȷ�𰸣�2Cl����2e�� = Cl2����ClO2������NaClO2����


 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵������ȷ����
A. ���õ����������к�ˮ����
B. ��ϩ������������������ֻ���ɻ������飨![]() �����÷�Ӧ������ɫ��ѧԭ�Ӿ�����Ҫ��
�����÷�Ӧ������ɫ��ѧԭ�Ӿ�����Ҫ��
C. ��ij��Һ�е��������ữ���Ȼ�����Һ���ְ�ɫ������֤������Һ��һ������SO42��
D. ���dzµ��㣬ԭ��֮һ�Ǵ������������������ζ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ó�����0.1mol/LijһԪ��HA��Һ��pH��1��0.1mol/LijһԪ��BOH��Һ��![]() ������������Һ�������Ϻ�������Һ�ʵĸ�����Ũ�ȼ��ϵ��ȷ���ǣ� ��
������������Һ�������Ϻ�������Һ�ʵĸ�����Ũ�ȼ��ϵ��ȷ���ǣ� ��
A��c (A��)��c (B��)��c (H��)��c (OH��)
B��c (B��)��c (A��)��c (H��)��c (OH��)
C��c (OH��)- c (H��)=c(HA)
D��c (B��)+ c (H��)=c (A��)+c (OH��) + c(HA)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�������������ɳ�����չ������ء�����˵������ȷ����
A.14C��������������ļ�����14C��12C��Ϊͬ��������
B.Ϊ��������ȱ��������ʳ�����м���һ�����ĵⵥ��
C.�ߴ��ȵ�SiO2�����Ƴɹ��أ�������ֱ��ת��Ϊ���ܣ�
D.Fe2O3��һ�ֺ���ɫ��ĩ����������ɫ�����Ϳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣����е�ÿ����ĸ��ʾһ�ֶ�����Ԫ��,�ش��������⣺
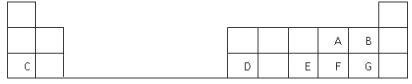
��1������C��ԭ�ӽṹʾ��ͼ__________��
��2��D��Ԫ�����ڱ��е�λ���ǵ������ڵ�________�塣
��3��A��B��E��F��G����Ԫ�����γɵ���̬�⻯����ȶ�����__________���ѧʽ����
��4��E��FԪ�ص�����������Ӧˮ��������Խ�ǿ����____________���ѧʽ����
��5��д��C��F�γɵĻ�����ĵ���ʽ______________________��
��6��A��C��D�ļ����Ӱ뾶��С��ϵ��__________________ �������ӷ��ű�ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ᾧ�����ɿɱ�ʾΪH2C2O4��xH2O��ij�о���ѧϰС������ͼװ�ý��������ᾧ�����ȷֽ�IJ��ֲ������֤����ʵ�顣��ش��������⡣

�����ϲ��ġ�
�����ᾧ����101 ��ʱ��ʼ�ۻ���150 ��ʱ��ʼ������175 ��ʱ��ʼ�ֽ⣻
������ƺͲ�����ƾ�Ϊ��ɫ�����
��1��������ͼ��ʾ��װ����ͨ��ʵ�������ᾧ��IJ��ַֽ������װ��B�пɹ۲쵽������ð���ҳ���ʯ��ˮ��������ɴ˼�ͬѧ�жϲ��ᾧ��ֽ�IJ�������CO2�����������ͬѧ�������䷴�Ե����ɿ�����______________________________________��
��2����ͬѧ��Ϊ���ᾧ��ֽ�IJ����к���CO��Ϊ������֤��XӦѡ��________(�ѧʽ)Ũ��Һ��װ��D��������____________________��
��3��ʵ��������漰���²������ٵ�ȼװ��A���ľƾ��ƣ���Ϩ��װ��A���ľƾ��ƣ��۵�ȼװ��E���ľƾ��ƣ���Ϩ��װ��E���ľƾ��ơ���4���������ȵ����˳��Ϊ____________(�����)����ȼE���ƾ���ǰ����Ҫ���еIJ�����______________��
��4��ʵ������з���װ��E�к�ɫ��ĩ���ɫ��װ��F���к�ɫ���������������װ��F�еĹ���Ϊ������������װ��F�з�����Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________��
��5����ͬѧ�õζ����ⶨ���ᾧ���нᾧˮ�ĺ��������������в�����
����һ���÷�����ƽ��ȡ3.15 g�����ĸò��ᾧ�������Ƴ�250 mL��Һ��
�����������Һ����ȡ25.00 mL���������Һ����ƿ�������������������ữ��
��������ȡ0.100 mol��L��1������KMnO4��Һ�����еζ������ν�����±���ʾ��
��һ�� | �ڶ��� | ������ | |
������Һ���(mL) | 25.00 | 25.00 | 25.00 |
����Һ���(mL) | 9.99 | 10.01 | 10.00 |
��֪�ζ���Ӧ�����ӷ���ʽΪ��MnO![]() ��H2C2O4��H���D��Mn2����CO2����H2O(δ��ƽ)��
��H2C2O4��H���D��Mn2����CO2����H2O(δ��ƽ)��
�����Ʋ�����Һ�IJ������������ǣ������������ձ�������ˮ�ܽ�������Һת����________��ϴ�ӣ����ݣ�ҡ�ȡ�
��ͨ������ȷ��x��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ���ǣ�������
A. �ɷǽ���ԭ���γɵĻ�����һ���ǹ��ۻ�����
B. ���ӻ���������Դ��ڹ��ۼ�
C. ���Ӽ������ۼ�����������ڻ�ѧ��
D. H2O��һ�ַdz��ȶ��Ļ�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ���ܹ��γɶ��ֻ������ش��������⣺
��1������(N2H4)������ΪҺ̬���ڿ�����Ѹ����ȫȼ������N2��ͬʱ�ų������ȣ���������������ɴ��������ȼ�ϡ�
��֪:H2(g)+1/2O2(g)==H2O(l)����H1=-285.8kJ/mol
N2(g)+2H2(g)=N2H4(l)����H2=+50.6kJ/mol
��N2H4(l)�ڿ���ȼ������Һ̬ˮ���Ȼ�ѧ����ʽΪ_____________��
(2)��ҵ�����ð�������������(HCN)�ķ�ӦΪCH4(g)+NH3(g)![]() HCN(g)+3H2(g) ��H>0��
HCN(g)+3H2(g) ��H>0��
��һ���¶��£���2L���������г���1mol CH4(g)��2mol NH3(g)����������Ӧ��4min�ﵽƽ��ʱ�����CH4��ת����Ϊ66.67%��0~4min�ȣ���H2��ʾ�ĸ÷�Ӧ����v(H2)=_____��
�����¶Ⱥ��ݻ����䣬�پ�ƽ���������г���2molNH3��2molH2����ʱv��___v��(ѡ�>����<����=��)��
��ƽ����ϵ��HCN�����ʵ���(n)��ij�������仯������ͼ��ʾ(ͼ��x��L�ֱ��ʾ�¶Ȼ�ѹǿ)��
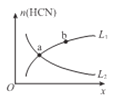
��xΪ�¶ȣ������ߣ�____(ѡ�L1����L2��)����ȷ��ʾn (HCN) ���¶ȵĹ�ϵ��
��xΪѹǿ��������____(ѡ�L1����L2��)����ȷ��ʾn HCN)��ѹǿ�Ĺ�ϵ��
(3)NH3�ܹ��γ�Ag(NH3)2+��
����Һ�д���Ag+ (aq)+2NH3 (aq)==Ag( NH3)2+(aq )ʱ����ƽ�ⳣ���ı���ʽΪK��=_______��
�������£�K��[Ag(NH3)2+]=1.10��107����ӦAgCl (s)+2NH3 (aq)![]() Ag( NH3)2+(aq) +Cl-(aq)�Ļ�ѧƽ�ⳣ��K=1.936��10-3����Ksp(AgCl)=_____��
Ag( NH3)2+(aq) +Cl-(aq)�Ļ�ѧƽ�ⳣ��K=1.936��10-3����Ksp(AgCl)=_____��
(4)��������͵��������dz����Ĵ�����Ⱦ�������ͼ��ʾ����(�缫��Ϊ���Ե缫)������SO2�����������ų�����Һ����NO2��
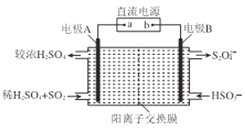
�ٵ缫A�ĵ缫��ӦʽΪ______________��
���ڼ��������£��������ų�����Һ����NO2��ʹ��ת��Ϊ�����塣ͬʱ��SO32-���ɡ��÷�Ӧ���ӷ���ʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ٻ����ͺ�ˮ���Һ��39%���Ҵ���Һ��ʹ���ǵ�ʳ��ˮ����壬�����ϸ���������ȷ���������ǣ� ��
A.��Һ�����ˡ�����
B.���ˡ�����Һ
C.��Һ��������
D.�����ˡ���Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com