
����Ŀ��ij��ʵ����Ԥ����Ҫʹ��480 mLamol/L�Ĵ�����Һ��������ijͬѧ��ʵ�������Ƹ���Һ������ͼ���ش��������⣺


(1)����ͼ�е����ƹ�������һ������ָ��ȱ�ٵIJ�����_____________��
(2)����ʵ�VֵΪ________���ɴ�ֵ�����a��________��ʹ������ƿǰ������ƿ������е�һ�������______________________��
(3)������������ͼ�����ֳ��IJ�������������������д����_____________��___________��____________________��
(4)ָ������ʵ������Խ���Լ�����������ҺŨ�ȵ�Ӱ�죬��д��ƫ������ƫС����������Ӱ������
��ת����Һʱ����ƿ��������������ˮ��________��
�ڶ���ʱ���ӿ̶��ߣ�________��
���𰸡�����ܢ�֮��ȱ��ҡ������ƿʹ��Һ��Ͼ��ȵIJ��� 500 1 ��������ƿ�Ƿ�©ˮ ����Na2CO3ʱ�Լ�λ�÷Ŵ��� ʹ�ý�ͷ�ζ�ʱ����� ����ʱ����λ�ò��� ��Ӱ�� ƫ��
��������
����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ���ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ƿ���й̶������������������������������Һ�����ʵ�����Ҫ����c=n/V��n=m/M���м��㼰����ʵ������в�������
(1)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ���ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���������Ϸ�����֪���ܢ�֮��ȱ��ҡ������ƿʹ��Һ��Ͼ��ȵIJ�����
�ʴ��ǣ�����ܢ�֮��ȱ��ҡ������ƿʹ��Һ��Ͼ��ȵIJ�����
(2)����ƿ���ɹ̶�����������û��480 mL����ƿ��ֻ��ѡ��500 mL����ƿ������ʵ�VֵΪ500����ȡ̼���ƹ���53g�����ʵ���Ϊ53g/106g/mol=0.5mol������c=n/V��֪��c=0.5mol/0.5L=1mol/L���ɴ�ֵ�����a��1��ʹ������ƿǰ������ƿ������е�һ������Ǽ�������ƿ�Ƿ�©ˮ��
�ʴ��ǣ�500��1����������ƿ�Ƿ�©ˮ��
(3)�������һ��Ũ�ȵ�ʵ��������裬������������ͼ�����ֳ��IJ����������������ٳ���Na2CO3ʱ�Լ�λ�÷Ŵ��ˣ�Ӧ��̼���Ʒ������̣���ʹ�ý�ͷ�ζ�ʱ����磬��Һ�����̶���1-2cmʱ����ʼ�ý�ͷ�ιܵμӣ��۶���ʱ����λ�ò��ԣ�Ӧ��ƽ�ӿ̶��ߣ����밼Һ�����У�
�ʴ��ǣ�����Na2CO3ʱ�Լ�λ�÷Ŵ��ˣ�ʹ�ý�ͷ�ζ�ʱ����磻����ʱ����λ�ò��ԣ�
(4)��ת����Һʱ����ƿ��������������ˮ����Ϊ������Һ���ջ�Ҫ��ˮ���ݣ���Ӱ�����ʺ���Һ���������������Һ��Ũ����Ӱ�죻
�ʴ��ǣ���Ӱ�죻
�ڶ���ʱ���ӿ̶��ߣ���Һ���ƫС��Ũ��ƫ��
�ʴ��ǣ�ƫ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��X��Y��Z��M��ԭ������������������X��Y��Z����Ԫ���У����γɺ�����Ԫ�ص�10������m��n��p��q�����з�Ӧm+n![]() p��+q��M������������Ӧ��ˮ����Ϊ��ǿ�ᡣ������˵������ȷ��
p��+q��M������������Ӧ��ˮ����Ϊ��ǿ�ᡣ������˵������ȷ��
A. �����Ӱ뾶X<Z<Y<M
B. ��X��Y��Z����Ԫ����ɵĻ�������������ӻ�����
C. X��Y��Z����Ԫ����ɵĻ������ˮ��Һһ��������
D. MZ2����������ˮ��ɱ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ��������������Ƶú�ʹ�ý���п�Ĺ��ҡ�һ������п��(ZnS������SiO2������FeS��CdS��PbS����)Ϊԭ���Ʊ�����п��������ͼ��ʾ��
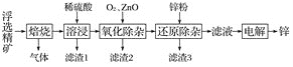
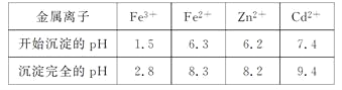
��ؽ�������[c0(Mn��)��0.1 mol��L��1]�γ��������������pH��Χ���£�
�ش��������⣺
(1)���չ�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ__________��
(2)����1����Ҫ�ɷֳ�SiO2���________���������ӹ�����ZnO��������_____________������ͨ��������������___________��
(3)��Һ�е�Cd2������п�۳�ȥ����ԭ���ӹ����з�Ӧ�����ӷ���ʽΪ__________��
(4)�������п��Һ�Ʊ�����пʱ�������ĵ缫��ӦʽΪ__________������п��ĵ��Һ�ɷ���________�������ʹ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�����ұ�![]() ����������������ϩ
����������������ϩ![]() ������˵���������
������˵���������
A. �ұ���ͨ��ʯ�ʹ��������
B. �ұ���ͬ���칹�峬������
C. ����ϩ���Ȼ��ⷴӦ�������ȴ�����ϩ
D. �ұ��ͱ���ϩ��������̼ԭ�Ӿ��ɴ���ͬһƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з����е�����ԭ���ӻ������������ͬ����( )
A. CO2��SO2 B. CH4��NH3 C. BeCl2��BF3 D. C2H2��C2H4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ɽ���Ԫ���������Ӧ���о���Ŀǰ��ѧ�о���ǰ��֮һ���Իش��������⣺
��1������������������ܽ�������Ⱦ���ȩ�������к�����ת��Ϊ������̼��ˮ���ﵽ�������йؼ�ȩ������������̼��ˮ˵����ȷ����_____��
�ٱ���B3N3H6���ܵ�������ȣ�
�ڼ�ȩ����������̼ԭ�Ӿ�����sp3�ӻ���
�۱���������̼��ˮ�ͼ�ȩ���ǷǼ��Է��ӣ�
��ˮ�ķе�ȼ�ȩ�ߵö࣬����Ϊˮ���Ӽ��ܴ����������ȩ���Ӽ䲻���������
��2��2007��ŵ��������ѧ��Ϊ������ѧ�Ұ������Ѷ��͵¹���ѧ�ұ˵ø��ֱ������ͬ��ã��Ա��������ھŵ���ЧӦ��CMRЧӦ���о�����ijɾͣ�ij���������������ͼ1������Aԭ��Ϊ�����Ķ��㣬Aλ������Ca��Sr��Ba��Pb����Bλ��V��Cr��Mn��Feʱ�����ֻ��������CMRЧӦ��
����A��B��O��ʾ�������⾧��Ļ�ѧʽ��_______����
��Cr��Mn�ĺ������������Ų�ʽ�ֱ�Ϊ��Cr��[Ar]3d54s1��Mn��[Ar]3d54s2�������ǵ�һ��������С���������˳���ǣ�_______�á��������У���
��3��CO2�Ŀռ乹��Ϊ______������CO2��SiO2�۷е����ܴ��ԭ����________
��4����������ˮ���ӵĿռ����з�ʽ����ʯ�������侧���ṹ��ͼ2�����ƣ����п�������ʾԭ��λ��������Ķ�������ģ�ʵ������ʾԭ��λ���������ڣ����ƣ�ÿ��������ƽ��ռ��__��ˮ���ӣ�����������ʯ���������з�ʽ��ͬ��ԭ����_______����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯�����ڿ�ѧ�о��ͻ����������������Ź㷺��Ӧ�á���ش��������⣺
��1���뵪Ԫ��ͬ��ĵ�������Ԫ�صĻ�̬ԭ�Ӽ۲���ӹ������ʽΪ___________��
��2�����ط��ӵĽṹ��ʽΪ��CO(NH2)2���÷�����������ĿΪ___________��ʵ���ã������е�����ԭ����ͬһƽ���ڣ������е�̼������125pm���ȵ��͵�̼��˫����121pm�������������е�̼������137pm���ȵ��͵�̼��������147pm���̣�˵�������д���____________��
��3�������ӹ���Ϊ_________���������У��������е�ÿ��H ������һ��������γɣ�1 mol��̬������_____mol�����
��4��ͨ����ΪCu3N�����Ӿ��壬�侧���ܿ�ͨ��ͼ(a)��Born-Haberѭ������õ���

��֪�� Cuԭ�ӵĵ�һ������Ϊ_____kJmol1��N��N������Ϊ_____kJmol1��Cu3N������Ϊ_____kJmol1��
��5��Cu3N����ľ�����ͼ(b)��ʾ�������߳�Ϊanm��������N3- λ��Cu+���γɵ����� ��������ģ�����������ı߳�Ϊ____nm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ���й����ʵ���Ũ�ȹ�ϵ��ȷ����
A��pH=2��HA��Һ��pH��12��MOH��Һ����Ȼ�ϣ�c(H+) + c(M+)>c(OH-) + c(A-)
B��pH��ȵ�CH3COONa��NaOH��Na2CO3������Һ��c(NaOH)��c(CH3COONa)��c(Na2CO3)
C�����ʵ���Ũ�����CH3COOH��CH3COONa��Һ�������ϣ�c(CH3COO-) +2c(OH-)��2c(H+) + c(CH3COOH)
D��0.1mol��L-1��NaHA��Һ����pH��4��c(HA-)��c(H+)��c(H2A)��c(A2-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������vtͼ������������ı�Կ��淴ӦA(g)��3B(g) ![]() 2C(g) ��H<0��Ӱ�졣�÷�Ӧ��������ʱ��Ĺ�ϵ��ͼ��ʾ��
2C(g) ��H<0��Ӱ�졣�÷�Ӧ��������ʱ��Ĺ�ϵ��ͼ��ʾ��

�ɼ���t1��t3��t5��t7ʱ��Ӧ���ﵽƽ�⣬���t2��t4��t6��t8ʱ��ֻ�ı���һ����Ӧ�����������ж�t2��t4��t6��t8ʱ�ı��������ж���ȷ����
A. ʹ���˴���������ѹǿ����С��Ӧ��Ũ�ȡ������¶�
B. �����¶ȡ���Сѹǿ����С��Ӧ��Ũ�ȡ�ʹ���˴���
C. ����Ӧ��Ũ�ȡ�ʹ���˴�������Сѹǿ�������¶�
D. �����¶ȡ���Сѹǿ������Ӧ��Ũ�ȡ�ʹ���˴���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com