
����Ŀ����һ���Ϊ1 L���ܱ������У�ͨ��һ������CO��H2O����850 �� �������·�Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��
CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��

(1)0��4 min��ƽ����Ӧ����v(CO)��__________________mol��(L��min)��1��
(2)850 ��ʱ��ƽ�ⳣ��K��___________________��
(3)850 ��ʱ������������г���1.0 mol CO��3.0 mol H2O����CO��ƽ��ת����Ϊ___________��
(4)���жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������________(��ѡ�����)��
a��v��(H2)��v��(H2O) b��c(CO2)��c(CO)
c�������������ܶȲ��� d��1 mol H��H�����ѵ�ͬʱ����2 mol H��O��
���𰸡�0.03 1 75% ad
��������
��1��0-4min��֪����c��CO��=0.20mol/L-0.08mol/L=0.12mol/L����v��CO��=![]() =
=![]() 0.03mol��Lmin��-1���ʴ�Ϊ��0.03��
0.03mol��Lmin��-1���ʴ�Ϊ��0.03��
��2��ƽ�ⳣ��K= ![]() =
= ![]() =1
=1
��3����һ����̼��ת��Ũ��Ϊx��
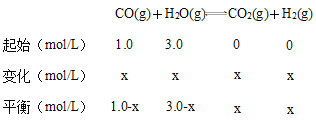
850��ʱ����Ӧ��ƽ�ⳣ����1����(1.0-x)��(3.0-x)=x2�����x=0.75����CO��ƽ��ת����Ϊ0.751.0��100%=75%��
��4���÷�Ӧ���ڹ̶��ݻ����ܱ������н��еķ�Ӧ���ҷ�Ӧǰ��������������䣬�ܶȲ�����Ϊ�ﵽƽ��ı�־��c(CO2)=c(CO)��һ�ض���״̬����һ���ﵽƽ�⣬a��v��(H2)=v��(H2O)��d��1mol H-H�����ѵ�ͬʱ����2mol H-O������Ϊ�ﵽƽ��ı�־��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N��Cu������ػ�������;�dz��㷺���ش��������⣺
��1����̬ͭԭ�ӵļ۵����Ų�ʽΪ__________________��
��2��ͭ��ش���ͬ������������������ͬ��ͭ���۷е㼰Ӳ�Ⱦ��ȼش���ԭ����___________________________��
��3��NH3���ӵ����幹��Ϊ_________������ԭ�ӵ��ӻ�������_________��
��4��N��S��P����ɵ����ʵij���Ԫ�ء�����Ԫ���е�һ������������_________���縺����С����_________��(��Ԫ�ط���)
��5����֪��Cu2O�۵�Ϊ1235�棬CuCl�۵�Ϊ426��������ж�Cu2OΪ_________ (�������Ӿ������������Ӿ���������ͬ)��CuClΪ_________��
(6)����ͭ�γɵ�һ�ֻ�����ľ����ṹ��ͼ��ʾ��
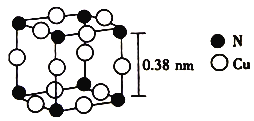
��ÿ��Cuԭ�ӽ��ڵ�Cuԭ����_________���������ӵ���������ֵΪNA���þ�����ܶ�Ϊ_________ (�г�����ʽ)g��cm��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǵڶ�����VA��Ԫ�أ��γɵ�N2H4��NH3�ȶ��ֺ������������Ź㷺����;���ش��������⣺
(1)�������ĺ�������Ų�ͼ��___________��
(2)����(N2H4)�ֳ���������������һ�ֿ�ȼ��Һ�壬��ȼ���Ƚϴ��Ҳ���Ի�������Ⱦ�����������ȼ�ϡ�
��֪��N2(g)+O2(g)=2NO(g) ��H1��
2NO(g)+O2(g)=2NO2(g) ��H2��
2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H3��
��Ӧ��N2H4(g)+O2(g)=N2(g)+2H2O ��H=____(�ú���H1����H2����H3�Ĵ���ʽ��ʾ)��
����������ȼ�ϵ����һ�ּ���ȼ�ϵ�أ���������Ⱦ���������Һ��20%��30%��KOH��Һ����������ȼ�ϵ�طŵ�ʱ�������ĵ缫��Ӧʽ��_____________������������Һ��pH________(����������������С������������)��
(3)ij�¶��£��ֱ����������Ϊ20 L���ܱ������г���1 mol N2��2.6 mol H2�ֱ𱣳ֺ��º��ݡ����º�ѹ�;��Ⱥ��ݣ���������Ӧ��N2(g)+3H2(g)![]() 2NH3(g) ��H<0����������(�ֱ���a��b��c��ʾ)��N2��ת����(��)�뷴Ӧʱ��(t)�Ĺ�ϵ��ͼ��ʾ��
2NH3(g) ��H<0����������(�ֱ���a��b��c��ʾ)��N2��ת����(��)�뷴Ӧʱ��(t)�Ĺ�ϵ��ͼ��ʾ��
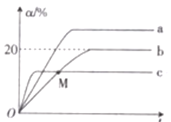
�����б�ʾ����ʱ��Ӧ�Ѵﵽƽ��״̬����______ (����ĸ)��
A. ������Ũ�Ȳ��ٱ仯 B. v(H2)=3v(N2)
C. ![]() ��ֵ���ٱ仯D. ��(N2)=16.7%
��ֵ���ٱ仯D. ��(N2)=16.7%
��ͼ�д�����Ӧ�ھ��Ⱥ��������н��е�������______(����a����b������c��)��
��b������M��v��_____(��������������������������)v����
������a�����������¸÷�Ӧ��ƽ�ⳣ��K=__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
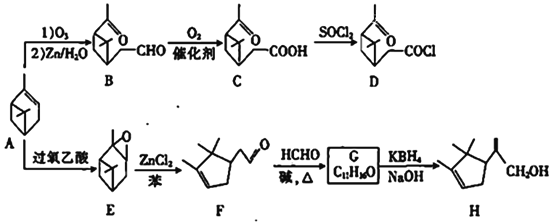
����Ŀ����A(��-��ϩ)Ϊԭ�Ͽ��Ʊ������м���D��H���ϳ�·�����£�
��֪����R1CHO+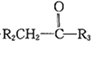
![]()
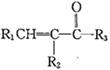 (R1��R2��R3ΪH������)
(R1��R2��R3ΪH������)
��һ�������£�R-C��C-OH�ɴ��ڡ�
�ش��������⣺
(1)A�еĹ�����������____________��E�ķ���ʽΪ___________
(2)C����D�ķ�Ӧ������___________
(3)̼ԭ��������4����ͬ��ԭ�ӻ����ʱ����̼��Ϊ����̼��E����________������̼��
(4)G�Ľṹ��ʽΪ___________
(5)H������Br2��CCl4��Һ��Ӧ�Ļ�ѧ����ʽΪ_____________________
(6)W��B��ͬ���칹�壬W��û�л�״�ṹ��һ�������£�W��ˮ������X��Y��X����NaHCO3��Ӧ����CO2����˴Ź�������ֻ������壬�����֮��Ϊ1��1��Y�ĺ˴Ź�������������壬�����֮��Ϊ9��6��1��W�Ŀ��ܽṹ��_______��.
(7)����ɱ����Ʊ�![]() �ĺϳ�·��(���Լ���ѡ)_________________��
�ĺϳ�·��(���Լ���ѡ)_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ú��Ϊȼ�Ͽ�ͨ����������;�����������
;����C��s��+O2��g���TCO2��g�� ��H1��0 ��
;�������Ƴ�ˮú����C��s��+H2O��g���TCO��g��+H2��g�� ��H2��0 ��
��ȼ��ˮú����2CO��g��+O2��g���T2CO2��g�� ��H3��0 ��
2H2��g��+O2��g���T2H2O��g�� ��H4��0 ��
��ش��������⣺
��1��;����ų�������������____��������������������������С������;����ų���������
��2��;��������ˮú���ķ�Ӧ���Ӧ�������е�������____�����������е���������������������������������С������������ڷ�Ӧʱ����Ӧ�����Ҫ___��������ת��Ϊ�����
��3��;��I��ͨ����ú����顢�������������ʵ������Ŀ�����ȼ�գ�����������Ŀ����______
��ʹú���ȼ�գ����������ת���� �ڼ���SO2�IJ��������������������
�ۼ����ж���CO������������Ⱦ���� �ܼ���CO2�IJ���������������ЧӦ��
��4����H1����H2����H3����H4����ѧ��ϵʽ��____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������AG����ʾ��Һ����ȣ������ʽΪ��AG=lg[![]() ]��298 Kʱ����0��1 mol/L��������Һ�ζ�10 mL 0.1 mol/L ��MOH��Һ���ζ�������ͼ��ʾ������˵����ȷ������ ��
]��298 Kʱ����0��1 mol/L��������Һ�ζ�10 mL 0.1 mol/L ��MOH��Һ���ζ�������ͼ��ʾ������˵����ȷ������ ��
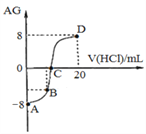
A. �õζ�ʵ�����ѡ���̪��Ϊָʾ��
B. C��ʱ����������Һ���������10 mL
C. �ζ������д�A�㵽D�㣬��Һ��ˮ�ĵ���̶�����С����������
D. ��B������������Һ���Ϊ5mL����������Һ�У�c(M+)+2c(H+)=c(MOH)+2c(OH)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Dz��ֶ�����Ԫ�ص�ԭ����������ij�ֳ������ϼ۵Ĺ�ϵͼ������ԭ��������������Ӧ��Ԫ�أ�������˵����ȷ����
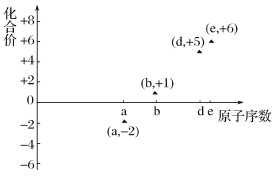
A.31d��33d����ͬ�ֺ���
B.��̬�⻯����ȶ��ԣ�a>d>e
C.��ҵ�ϳ��õ�ⷨ�Ʊ�����b
D.a��b�γɵĻ����ﲻ���ܺ����ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ý(V2O5)�Ǵ����������õĴ�����Ϊ�ۺ����ã�������Ա����������һ�����ӽ��������շϷ����¹��գ������ʴ�90%���ϡ���֪�Ϸ������к���V2O5��VOSO4�������Բ������������Ͽ�֪��VOSO4������ˮ��V2O5������ˮ��NH4VO3������ˮ���ù��յ���������ͼ��

��1��ˮ��ʱ��Ϊ����߷Ϸ��Ľ����ʣ����˷��飬�����Բ�ȡ�Ĵ�ʩ��___________________________________��__________________________________��д��2������
��2��д����Ӧ�ٵ����ӷ���ʽ_______________________________________________��
��3���ù����з�Ӧ�۵ij������ǻ��շ��Ĺؼ��������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ��ϵ����NH4Cl������������Һ��V2O5�������ȣ����¶ȡ�������ͼ��������ʵ��Ȼ��ϵ�����¶ȣ��Ȼ��ϵ��Ϊ___________���¶�Ϊ_____________��

��4����Ӧ�ڷ�������Һ�е���Ԫ����Cl������ʽ���ڣ���д����Ӧ�ڵĻ�ѧ����ʽ__________________________________________________________________��
��5���������ϵ�֪��NH4VO3Ҳ��ƫ����泥�����Է�����Ϊ117��20��ʱ��NH4VO3�ܽ��Ϊ0.468g����֪���ӽ�������Һ��c��VO3����=0.10mol/L����Ӧ�۳���ʱ����������0.10mol/L��NH4Cl��Һ��ͨ����ʽ�����жϴ�ʱ�Ƿ���NH4VO3��������������Һ����仯���Բ��ƣ�____________
��6��д�������շ�Ӧ��������NH4VO3�Ʊ�V2O5�Ļ�ѧ����ʽ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����¯�����з����Ļ�����Ӧ֮һ���£�
FeO���̣���CO������Fe���̣���CO2��������H��0
��1����÷�Ӧ��ƽ�ⳣ������ʽK��_________________
��2����֪1100��ʱK��0.263���¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��¯��CO2��CO�������ֵ_______ƽ�ⳣ��Kֵ___����������С�䣩.
��3��1100��ʱ��ø�¯��c(CO2)��0.025mol��L��1��c(CO)��0.1mol��L��1������������£��÷�Ӧ�Ƿ���ƽ��״̬________��ѡ���ǻ����ʱ��ѧ��Ӧ����v��____v����ѡ�������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com