
����Ŀ����A������ͼ�У��ʺɱ�������ֵΪ42��̼����Ԫ�ص�������Ϊ6:1����˴Ź��������������壬��������Ϊ1:2:3��A�������л���֮��Ĺ�ϵ���£�

��֪��CH2��CH2![]() HOCH2CH2OH���ش��������⣺
HOCH2CH2OH���ش��������⣺
(1)�л���B�ķ���ʽ___________________________��
(2)�߾���F�ṹ��ʽΪ___________________��
(3)д��C�����Ƶ�������ͭ��Ӧ�Ļ�ѧ����ʽ___________________________��
(4)E��һ�������¿������Ӧ����һ����Ԫ���л���H��H�Ľṹ��ʽ________.��
(5)д������G�Ļ�ѧ����ʽ_____________________________________________��
���𰸡� ��C3H8O2 ![]()
![]()
 n
n![]()
![]()
![]() +��n-1��H2O
+��n-1��H2O
��������������������⿼���л��ƶϣ��漰�л������ʽ�ͽṹ��ʽ��ȷ�����л������ʽ�ͽṹ��ʽ����д���л���Ӧ����ʽ����д��A������ͼ���ʺɱ�������ֵΪ42��A����Է�������Ϊ42����A��n��C����n��H��=![]() ��
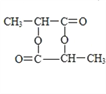
�� ![]() =1:2��A��ʵ��ʽΪCH2��A�ķ���ʽΪ��CH2��x��14x=42�����x=3��A�ķ���ʽΪC3H6��A�ĺ˴Ź����������������ҷ�������Ϊ1:2:3��A�Ľṹ��ʽΪCH2=CHCH3��A�����Ӿ۷�Ӧ���ɵĸ߾���F�Ľṹ��ʽΪ
=1:2��A��ʵ��ʽΪCH2��A�ķ���ʽΪ��CH2��x��14x=42�����x=3��A�ķ���ʽΪC3H6��A�ĺ˴Ź����������������ҷ�������Ϊ1:2:3��A�Ľṹ��ʽΪCH2=CHCH3��A�����Ӿ۷�Ӧ���ɵĸ߾���F�Ľṹ��ʽΪ![]() ��A��B���������֪�ķ�Ӧ��B�Ľṹ��ʽΪ
��A��B���������֪�ķ�Ӧ��B�Ľṹ��ʽΪ![]() ��B��C�������Ĵ�������C�Ľṹ��ʽΪ
��B��C�������Ĵ�������C�Ľṹ��ʽΪ![]() ��C��Cu��OH��2����ʱ��C��-CHO���������ữ��õ���D�Ľṹ��ʽΪ
��C��Cu��OH��2����ʱ��C��-CHO���������ữ��õ���D�Ľṹ��ʽΪ![]() ��D��H2�����ӳɷ�Ӧ����E��E�Ľṹ��ʽΪ
��D��H2�����ӳɷ�Ӧ����E��E�Ľṹ��ʽΪ![]() ��E�к��ǻ����Ȼ���E�������۷�Ӧ���ɸ߾���G��G�Ľṹ��ʽΪ
��E�к��ǻ����Ȼ���E�������۷�Ӧ���ɸ߾���G��G�Ľṹ��ʽΪ![]() ��
��
��1��B�Ľṹ��ʽΪ![]() ��B�ķ���ʽΪC3H8O2��
��B�ķ���ʽΪC3H8O2��
��2���߾���F�Ľṹ��ʽΪ![]() ��
��
��3��C�Ľṹ��ʽΪ![]() ��C������Cu��OH��2��Ӧ�Ļ�ѧ����ʽΪ
��C������Cu��OH��2��Ӧ�Ļ�ѧ����ʽΪ![]() +2Cu��OH��2+NaOH
+2Cu��OH��2+NaOH![]()
![]() +Cu2O��+3H2O��
+Cu2O��+3H2O��
��4��E�Ľṹ��ʽΪ![]() ��2����Eͨ��������Ӧ�γ���Ԫ���л���H��H�Ľṹ��ʽΪ
��2����Eͨ��������Ӧ�γ���Ԫ���л���H��H�Ľṹ��ʽΪ ��
��
��5��G��E�������۷�Ӧ���ɣ�����G�Ļ�ѧ����ʽΪn![]()
![]()
![]() +��n-1��H2O��
+��n-1��H2O��
�����͡��ƶ���
��������
18
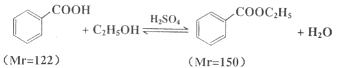
����Ŀ��������������C9H10O2������ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��塢�ܼ��ȡ����Ʊ�����Ϊ��
��֪��
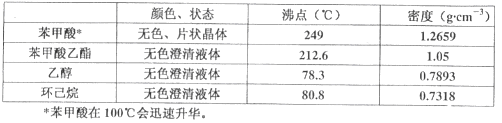

ʵ�鲽�����£�����100 mLԲ����ƿ�м���12.20 g�����ᡢ25mL�Ҵ�����������20mL�����飬�Լ�4mLŨ���ᣬ��Ͼ��Ȳ������ʯ��������ͼ��ʾװ�������������¶���65��70����Ȼ���2h����Ӧʱ������-�Ҵ�-ˮ���γɡ���������е�62.6�棩��������������÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���
�ڷ�Ӧ�������������ų���ˮ����Һ��ر��������������ȣ�����ˮ�����ռ�����Һ�岻���������ӣ�ֹͣ���ȡ�
�۽���ƿ�ڷ�ӦҺ����ʢ������ˮ���ձ��У���������Na2CO3����Һ�����ԡ�
���÷�Һ©���ֳ��л��㣬ˮ����25mL������ȡ��Һ��Ȼ��ϲ��л��㡣�����Ȼ��ƣ��Դֲ�Ʒ��������װ����ͼ��ʾ���������������Ѻ�������£�����210��213�����֡�
�ݼ���ϸ�ò�Ʒ���Ϊ12.86mL.
(1)�������ʹ�÷�ˮ�����Ϸ����ȥˮ��Ŀ����_________________��
(2)�������Ӧ������ֵ��¶���___________________��
A��65��70�� B��78��80�� C��85��90�� D��215��220��
(3)���������Na2CO3���벻�㣬�ڲ��������ʱ��������ƿ�пɼ����������ɣ������������ԭ����_____________��
(4)������з�Һ����������ȷ����__________��
A.ˮ��Һ�м������ѣ�ת������Һ©���У����ϲ�����������Һ©����ת������ҡ
B.��ҡ���κ����Һ©���¿ڵIJ���������
C.��������ҡ���������ֳַ�Һ©�����ô�Һ��ֲ�
D.��Һ����ʱ����Һ©���е��²�Һ�����¿ڷų���Ȼ���ٽ��ϲ�Һ�����¿ڷų�
����װ��ͼ������A��������___________���ڲ�����м����Ȼ��Ƶ�������_________��
(5)��ʵ���Ʒ�IJ���Ϊ____________��
���𰸡� ������ƽ�ⲻ�����������ƶ�����߱������������� C �����������л��б����ᣬ��������100��ʱ�������� AB ������ƿ ��ˮ�� 90.02%
��������������������⿼�鱽�����������Ʊ���
��1����Ӧ![]() +CH3CH2OH
+CH3CH2OH![]()
![]() +H2OΪ���淴Ӧ��ʹ�÷�ˮ�����Ϸ����ȥˮ����С������Ũ�ȣ�������ƽ�ⲻ��������Ӧ�����ƶ�����߱����������IJ��ʡ�
+H2OΪ���淴Ӧ��ʹ�÷�ˮ�����Ϸ����ȥˮ����С������Ũ�ȣ�������ƽ�ⲻ��������Ӧ�����ƶ�����߱����������IJ��ʡ�
��2���������⣬��Ӧʱ������-�Ҵ�-ˮ���γ����������������������ƿ�ڵı����������л����Ҵ��������顢������������������������������������������е��Ҵ��������飬�Ҵ��ķе�Ϊ78.3����������ķе�Ϊ80.8���������������ķе�Ϊ212.6�������Բ�����Ӧ������ֵ��¶���85~90������ѡC��
��3���������м���Na2CO3��ȥ�����������л��еı���������ᣬ��Na2CO3���벻�㣬������û����ȫ��ȥ������������ʱ������ƿ�пɼ����̵�ԭ���ǣ������������л��б���������������������100��ʱ����������
��4��A��Ϊ��ʹ���Ѻ�ˮ��Һ��ֽӴ���ˮ��Һ�м�������ת������Һ©���к������ϲ�����������Һ©����ת������ҡ��A����ȷ��B��Ϊ��ֹ��Һ©������ѹ����������������ҡ���κ����Һ©���¿ڵIJ�����������B����ȷ��C����������ҡ���������轫��Һ©����������̨�Ͼ��á���Һ��ֲ㣬C�����D����Һ����ʱ����Һ©���е��²�Һ�����¿ڷų���Ȼ���ϲ�Һ����Ͽ��㵹������D�����ѡAB������װ��ͼ������A��������������ƿ���ڲ������м���CaCl2����������Ϊ��ˮ������ȥˮ��
��5�������Ҵ��������Ա���������������ɵı�����������![]() ~
~![]() ��n������������������=n�������ᣩ=
��n������������������=n�������ᣩ=![]() =0.1mol��m������������������=0.1mol
=0.1mol��m������������������=0.1mol![]() 150g/mol=15g����ʵ���Ʒ�IJ���=
150g/mol=15g����ʵ���Ʒ�IJ���=![]() 100%=90.02%��
100%=90.02%��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�����Ȼ��ƹ��塢̼������Һ��ϡ������Լ������Ƶ�̼��ƣ������Ƶô���������ƾ��塣
(1)д����ȡ�����з�Ӧ�����ӷ���ʽ��________________��________________________��
(2)�������ͬѧ�������ʵ���������(��Ҫ��ش�ʹ�õ�����)
��������ˮ��ȫ�ܽ�CaCl2����________��
�ڽ���Ӧ��Ļ������ˣ�������������ˮϴ�ӳ�������Cl����
�ۼ���________________��ʹ������ȫ�ܽ⡣
�ܽ�������Һ�������ᾧ���õ�����������ƾ��塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���һ����Ҫ���л�ԭ�ϣ��ڴ����������£�CO��H2��Ӧ�����ɼ״� (CH3OH) ������CH4����Ӧ������
��Ӧ��CO(g)+2H2(g)![]() CH3OH(g) ��H1=-90.0kJ/mol
CH3OH(g) ��H1=-90.0kJ/mol
�� Ӧ��CO(g)+3H2(g)![]() CH4(g) + H2O(g) ��H2
CH4(g) + H2O(g) ��H2
��Ӧ�� CH4(g)+2H2O(g)![]() CO2(g)+ 4H2(g) ��H3=+125.0 kJ/mol
CO2(g)+ 4H2(g) ��H3=+125.0 kJ/mol
��Ӧ��CO(g)+ H2O(g)![]() CO2(g) + H2(g) ��H4=-25.0 kJ /mol
CO2(g) + H2(g) ��H4=-25.0 kJ /mol
K1��K2��K3��K4�ֱ��ʾ��Ӧ����������������ƽ�ⳣ����
�ش�����������
��1����Ӧ����ƽ�ⳣ���ı���ʽΪK2=______________��K2��K3��K4�Ĺ�ϵΪK2=______________����H2=____________kJ/mol��
��2��ͼ1������ȷ��ʾ��Ӧ����ƽ�ⳣ��(lgK1) ���¶ȱ仯������Ϊ______________����������ĸ�������ж�����Ϊ______________________________________________________________��

��3�����º��ݵ������£����������˵����Ӧ���ﵽƽ��״̬����__________________��
A.2v�� (H2)=v��(CH3OH) B.���������ܶȲ��ٸı�
C.��������ƽ��Ħ���������ٸı� D.��������ѹǿ���ٸı�
��4��Ϊ̽����ͬ������CO��H2����CH3OH��ѡ����Ч����ijʵ���ҿ���CO��H2�ij�ʼͶ�ϱ�Ϊ1��3����ʵ�飬�õ�����������
T/K | ʱ��/min | �������� | �״��ĺ���(%) |
450 | 10 | CuO-ZnO | 78 |
450 | 10 | CuO-ZnO-ZrO2 | 88 |
450 | 10 | ZnO-ZrO2 | 46 |

���ɱ�1��֪����Ӧ������Ѵ���Ϊ______________��ͼ2��a��b��c��d�ĵ��Ǹ��¶���COƽ��ת���ʵ���_________________________________��
�����������COת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��_________________��
A.ʹ�ô���CuO-ZnO-ZrO2 B.�ʵ����ͷ�Ӧ�¶�
C.����CO��H2�ij�ʼͶ�ϱ� D.�����£��ٳ���a molCO��3a mol H2
��5����֪1000������ӦCO(g)+ H2O(g)![]() CO2(g) + H2(g) K4=1.0�����¶��£���ijʱ����ϵ��CO��H2O��CO2��H2��Ũ�ȷֱ�Ϊ3molL-1��1molL-1��4molL-1��2molL-1�����ʱ������Ӧ��v��(CO)_______v��(CO) �����������������=�����ﵽƽ��ʱc(CO)=___________ molL-1��
CO2(g) + H2(g) K4=1.0�����¶��£���ijʱ����ϵ��CO��H2O��CO2��H2��Ũ�ȷֱ�Ϊ3molL-1��1molL-1��4molL-1��2molL-1�����ʱ������Ӧ��v��(CO)_______v��(CO) �����������������=�����ﵽƽ��ʱc(CO)=___________ molL-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ij�ݻ��̶����ܱ������ɿ��ƶ��Ļ������� A��B ���ң��� A �г���һ���� H2��O2 �Ļ�����壬�� B �г��� 1 mol ��������ʱ������λ����ͼ��ʾ��

��1��A �һ����������ʵ���Ϊ______��������������Լ______��
��2��ʵ���� A �һ�����������Ϊ 34 g����û��������ܶ���ͬ��ͬѹ�����º����ܶȵ�______����
��3������ A �� H2��O2�Ļ�������ȼ�������ָ�ԭ�¶Ⱥ����ջ���ͣ����λ����______�̶ȣ�����������ѹǿ�뷴Ӧǰ����ѹǿ֮��Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ѿ�̫���ܵ���ǽ����������о��ȵ㣬�߱�������ࡢ����Ӧ�á�����ɱ��ͺ�Ч�ʸߵ������ŵ㣬����һ�ָ��ѿ�̫���ܵ�ز��ϵľ�����ͼ���ش��������⣺
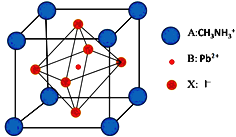
��1��Ǧ��Ǧ�ε���ɫ��ӦΪ��ɫ�������й�ԭ��������������ȷ����_________(����ĸ)��
a.���Ӵӻ�̬ԾǨ���ϸߵļ���̬ b.���Ӵӽϸߵļ���̬ԾǨ����̬
c.��ɫ��Ӧ�Ĺ����������չ��� d.��ɫ��Ӧ�Ĺ������ڷ������
��2��̼ԭ�Ӽ۲���ӵĹ������ʽ�������Ų�ͼ��Ϊ_________����̬Pbԭ�Ӻ�������Ų������ռ���ܼ��ĵ���������ͼ��״Ϊ___________��
��3��CH3NH3+�к��л�ѧ����������________������ĸ��ţ���Nԭ�ӵ��ӻ���ʽΪ______����CH3NH3+��Ϊ�ȵ�����ķ���Ϊ_________
a.���Լ� b. �Ǽ��Լ� c.��λ�� d. ���Ӽ� e.�Ҽ� f.�м�
��4��NH4+��H��N��H�ļ��DZ�NH3��H ��N��H�ļ��Ǵ��ԭ����__________��NH3��ˮ������ͭ�����γɵĻ������������ӳ����������İ�����ṹ(����ͼ)���û��������ʱ����ʧȥˮ�����ԭ�ӽṹ�Ƕȼ��Է�����__________��
��5����I- ���ڵ�I- ����Ϊ__________��X��������ʵ���þ����������ܶ�Ϊa g��cm-3�����ı߳�Ϊ____________pm�������ʵ����ԭ������ΪM��NA��ʾ�����ӵ�������ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵����ȷ����
A����2H��18O����ɵ�ˮ11 g��������������Ϊ4NA
B��1 mol N2��4 mol H2��Ӧ���ɵ�NH3������Ϊ2NA
C����״���£�7.1 g����������ʯ�����ַ�Ӧת�Ƶ�����Ϊ0.2NA
D��NO2��H2O��Ӧÿ����2 mol HNO3ʱת�Ƶĵ�����ĿΪ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ���������������
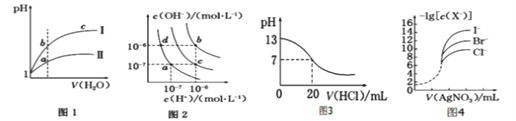
A. ͼ1��ʾͬ�¶��£�pH��1 ������ʹ�����Һ�ֱ��ˮϡ��ʱpH �ı仯���ߣ�����������Ϊ���ᣬ��a ����Һ�ĵ����Ա�b ��ǿ
B. ͼ2 �д�ˮ�������¶ȣ�����ʹa��䵽c��
C. ͼ3 ��ʾ25 ��ʱ����0.100 0 molL��1HCl �ζ�20 mL 0.100 0 molL��1NaOH ��Һ����Һ��pH�������������ı仯
D. ��0.010 0 molL��1AgNO3����Һ�ζ�Ũ�Ⱦ�Ϊ0.100 0 molL��1Cl����Br����I���Ļ����Һ����ͼ4 ���ߣ���ȷ�����ȳ�������I��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й�H��D��T��HD��H2��D����H������������˵����ȷ����
A.��Ϊͬλ��B.��������Ԫ��
C.HD��H2���ǵ���D.��������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Ԫ��֮һ������ֲ���纣���������к��зḻ�ĵ�Ԫ�ء��غ���������ʳ�ú�������ˣ���״���״�ȵ�ȱ���������ʵ͡���֪����Ԫ���Ե����ӵ���ʽ���ڣ�������I���ܱ���ˮ����ΪI2��I2���л��ܼ��е��ܽ�����Դ�����ˮ�е��ܽ�ȣ�I2����ɫ����I������ɫ��ʵ������Ӻ�������ȡ���������ͼ��ʾ��
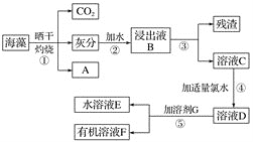
��1��д��A�Ļ�ѧʽ______________��
��2������۲�������Ҫ�IJ���������______________��______________��__________________��
��3�������л��ܼ��в�����Ϊ�ܼ�G����________(����������)��
A���ƾ�����B�����Ȼ�̼����C�����ᡡ��D.���͡���E.��
��4������ݵIJ���������__________����Һ����ҺE����ɫ����ҺF����ɫ______(����dz��)��
��5��������У���ѡ�ã�3���е�__________(����Һ����)Ϊ�ܼ�G����Һʱ��Ӧ�Ȱ���Һ____(�E����F��)�ӷ�Һ©�����²��ų���֮���ٰ���һ��Һ�ӷ�Һ©�����Ͽڵ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com