
����Ŀ����ѧ��Ӧ���������仯������(C3H8)��һ��������ȼ�ϣ���ͼ��һ���������ڳ��³�ѹ����ȫȼ������CO2��1 mol H2O(l)�����е������仯ͼ���Իش��������⣺
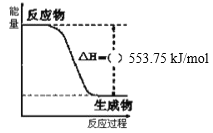
(1)д������ȼ���ȵ��Ȼ�ѧ����ʽ��___��
(2)������(CH3OCH3)��һ������ȼ�ϣ�Ӧ��ǰ��������1 mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455 kJ����������1 mol����Ͷ����ѵĻ��������ȫȼ������CO��Һ̬ˮ���ų�1607 kJ�����������������б���Ͷ����ѵ����ʵ���֮��Ϊ___��
���𰸡�C3H8(g)+5O2(g)=3CO2(g)+4H2O(l) ��H= -2215.0 kJ/mol 1��4
��������
(1)����ȼ�շ�Ӧ�Ƿ��ȵģ�������H<0������ͼ��õ�����1 molˮ���ʱ���H=-553.75 kJ/mol�������Ȼ�ѧ����ʽ��д����д����ע�����ʾۼ�״̬����Ӧ���µ��ʱ䣻
(2)�����Ȼ�ѧ����ʽ��ϻ���������ʵ����ͷ�����ʽ����õ������Ѻͱ������ʵ���֮�ȡ�
(1)ͼ����һ����������ȫȼ������CO2��1 mol H2O(l)�����е������仯ͼ������ȼ�շ�Ӧ�Ƿ��ȵģ�������H=-553.75 kJ/mol��������ȫȼ������CO2��1 mol H2O(l)��Ӧ������H= -553.75 kJ/mol�����ڱ��黯ѧʽ��C3H8����1 mol������ȫȼ�ղ���4 mol H2O����Ӧ�ų�����Q=4 mol��553.75 kJ/mol=2215.0 kJ/mol���ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ��C3H8(g)+5O2(g)=3CO2(g)+4H2O(l) ��H=-2215.0 kJ/mol��
(2)1 mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455 kJ������1 mol������ȫȼ�շų�2215.0 kJ����������1 mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1607 kJ��������1 mol��������ж��������ʵ���x���������ʵ���Ϊ(1-x)��1455x kJ+2215.0(1-x) kJ=1607 kJ�����x=0.8�����������б������ʵ���Ϊ0.2 mol���ʻ�������б���Ͷ��������ʵ���֮��n(C3H8)��n(CH3OCH3)=0.2 mol��0.8 mol=1��4��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��װ��a��b��c�зֱ�ʢ���Լ�1��2��3������ͼ��ʾ��װ�ý���ʵ��(�г�������ȥ����Ҫʱ�ɼ���)���ܴﵽ��Ӧʵ��Ŀ�ĵ���
ѡ�� | �Լ�1 | �Լ�2 | �Լ�3 | ʵ��Ŀ�� | װ�� |
A | Ũ | CuƬ | KI-������Һ | ��֤ |
|
B | ���� | ʯ��ʯ | ���� | �Ʊ� | |
C | ϡ���� | ��ҺX | ����ʯ��ˮ | ��֤��ҺX���Ƿ��� | |
D | 70%���� |
| ���� | ֤�� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ú�Ľྻ���������������������������ࣩ����Ч����ȼú������SO2�ĺ������ѳ�Ϊ�ҹ���������������������֮һ��ͨ����������������Ԫ���Թ�����ʽ����úȼ�յIJ����С�ʯ��ʯ�dz��õĹ����������������漰�IJ��ַ�Ӧ���£�
��CaCO3(s)![]() CaO(s) + CO2(g) ��H1= +178.30kJ/mol
CaO(s) + CO2(g) ��H1= +178.30kJ/mol
��CaO(s) + SO2(g) + 0.5O2(g)![]() CaSO4(s) ��H2= -501.92 kJ/mol
CaSO4(s) ��H2= -501.92 kJ/mol
��CO(g) + 0.5O2(g) ![]() CO2(g) ��H3
CO2(g) ��H3
��CaSO4(s) + CO(g) ![]() CaO(s) + SO2(g) + CO2(g) ��H4= +218.92kJ/mol
CaO(s) + SO2(g) + CO2(g) ��H4= +218.92kJ/mol
��1���¶����ߣ���Ӧ�ٵĻ�ѧƽ�ⳣ��________���������С�����䡱����
��2����H3=________kJ/mol��
��3����úȼ�չ����г������Թ����Ŀ�������߹����ʣ�ȼ�ղ�������Ԫ�ص�����ռȼú����Ԫ���������İٷֱȣ�����Ϸ�Ӧ�ڡ��ۡ��ܷ�����ԭ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ijһԪ����HA�ĵ��볣��K��1.6��10��6����20.00 mL Ũ��ԼΪ0.1 mol��L��1 HA��Һ����μ���0.1000 mol��L��1�ı�KOH��Һ����pH�仯������ͼ��ʾ(�����¶ȱ仯)����ش������й����⣺(��֪lg4��0.6)
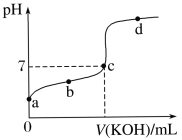
��1��a����Һ��pHԼΪ________����ʱ��Һ��H2O�������c(H��)Ϊ________��
��2��a��b��c��d�ĵ���ˮ�ĵ���̶�������________�㣬�ζ���������ѡ��__________��ָʾ�����ζ��յ���________(�c�����ϡ���c�����¡�)��
��3���ζ������в��ֲ������£����и�����ʹ�������ƫ�ߵ���__________������ĸ��ţ���
A���ζ�ǰ��ʽ�ζ���δ�ñ�KOH��Һ��ϴ
B��������ˮϴ����ƿ������װ��HA��Һ����еζ�
C���ζ������У���Һ���ֱ�ɫ������ֹͣ�ζ�
D���ζ�����������Һ�棬��ȡKOH��Һ���
��4�����ظ����εζ�ʵ����������±���ʾ������ζ�����HA��Һ�����ʵ���Ũ��Ϊ____mol/L��ע����Ч���֣���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Īɳ������һ����ʹҩ�����ĺϳ�·�����£�
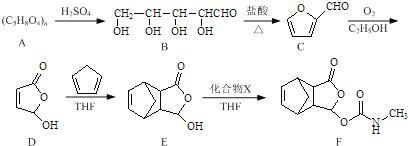
��1��B������̼ԭ����Ϊ____________��������D�к��������ŵ�����Ϊ____________��
��2��C������������ͭ��Ӧ�Ļ�ѧ����ʽΪ____________��
��3��д��ͬʱ��������������E��һ��ͬ���칹��Ľṹ��ʽ��____________
I���˴Ź���������4���壻
�����ܷ���������Ӧ��ˮ�ⷴӦ��
��������FeCl3��Һ������ɫ��Ӧ��
��4����֪E+X��FΪ�ӳɷ�Ӧ��������X�Ľṹ��ʽΪ____________��
��5����֪��![]() ��������
��������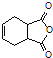 �Ǻϳɿ�����ҩ������Τ���м��壬����ƺ���������
�Ǻϳɿ�����ҩ������Τ���м��壬����ƺ���������![]() ��
��![]() Ϊԭ�Ϻϳɸû�����(�úϳ�·������ͼ��ʾ����ע����Ӧ����)��______________��
Ϊԭ�Ϻϳɸû�����(�úϳ�·������ͼ��ʾ����ע����Ӧ����)��______________��
�ϳ�·������ͼʾ�����£�![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ���ں����ܱ������������з�Ӧ�ϳɼ״���CO��g��+2H2��g��![]() CH3OH��g����H
CH3OH��g����H
��1���÷�Ӧ��ƽ�ⳣ������ʽΪ_______��
��2��������������Ƿ�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K��
�¶� | 250�� | 300�� | 350�� |
K | 2.041 | 0.270 | 0.012 |
���ɱ��������жϸ÷�Ӧ�ġ�H______0�����������=����������
��ij�¶��£���2molCO��6molH2����2L���ܱ������У���ַ�Ӧ10s��ﵽƽ��ʱ���c��CO��=0.2mol/L����CO��ת����Ϊ____����H2��ʾ��Ӧ����Ϊ_____����ʱ���¶�Ϊ______��
��3��Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��______��
a������ b��������� c������CO��Ũ��
d�����ݳ���H2 e����ѹ����������� f��������״�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣�ÿ����Ŵ���һ��Ԫ�أ������Ҫ��ش����⣺
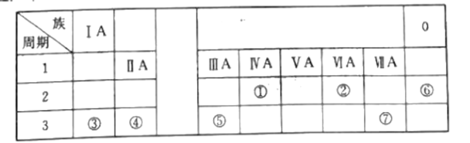
��1�����н�������ǿ��Ԫ��Ϊ___����Ԫ�ط��ţ���
��2�����ȶ���Ԫ��Ϊ___����Ԫ�ط��ţ���
��3���ۡ��ߵ�ԭ�Ӱ뾶��С��ϵ����___(����>����<������=��)�ߣ���
��4���ܺ͢�����Ԫ����ɵĻ�������AgNO3��Һ��Ӧ�����ӷ���ʽΪ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����«��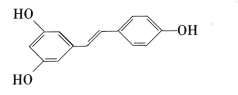 �㷺������ʳ��(��ɣ�ء�����������������)�У������п����ԡ���ش��������⡣
�㷺������ʳ��(��ɣ�ء�����������������)�У������п����ԡ���ش��������⡣
��1����«���ķ���ʽΪ_____�����������ŵ�����Ϊ_____��
��2�����й��ڰ�«����˵����ȷ����_____
A.��ʹ����KMnO4��Һ��ɫ
B.����FeCl3��Һ��Ӧ����ɫ
C.��ʹ���CCl4��Һ��ɫ
D.����NH4HCO3��Һ��Ӧ��������
E.���ڴ���
F.���ܷ���������Ӧ
��3��1mol��������������____molNaOH�����������_____molBr2��
��4��1mol���л�����H2�ӳ�ʱ��������ı�״���µ�H2�����Ϊ_____L��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com