
����Ŀ��ijͬѧ��������ͭ����(![]() )�ᾧˮ�����IJⶨʵ�顣���������գ�
)�ᾧˮ�����IJⶨʵ�顣���������գ�
��ʵ�鲽�裩��
(1)��__________(����������)ȷ������������������
(2)�ڴ������м���һ����������ͭ���壬�����ء�
(3)��ʢ������ͭ����Ĵ������������������������ȣ�ֱ����ɫ��ȫ��ף�Ȼ�����������_______(����������)����ȴ�����£������ء�
(4)�ظ�����ʵ����к��ز�������Ŀ����_______________��ֱ�����γ������������______�ˡ�
(5)�����Ǹ�ѧ��ʵ���һ�����ݣ�����ɼ��㣺
��������(��) | �����뾧�������(��) | ���غ�������������� |
13.721 | 24.692 | 20.631 |
![]() ______��(��ȷ��0.01)ʵ����������_________(����С����һλ)��
______��(��ȷ��0.01)ʵ����������_________(����С����һλ)��
(6)���ʵ���в�������ԭ�������_______
a. ����ͭ�����к��в��ӷ������� b. �ڼ��ȹ����з����к�ɫ��������
c. ����ʱ�о���ɽ����� d. ����ʧˮ��¶���ڿ�������ȴ
���𰸡�������ƽ ������ ȷ���ᾧˮʧȥ��ȫ 0.001 5.22 4.4% bc
��������
��1�����ݾ�ȷ��ѡ�����������
��3���ڼ��Ⱥ���ȴʱ��Ϊ�˷�ֹ����ͭ��ˮ��Ӧ������ͭ���ڸ������н�����ȴ���Ӷ��õ��������������ˮ����ͭ��
��4��ȡ����ƽ��ֵ������������Ϊȷ������ͭ������ȫʧȥ�ᾧˮ���������ȴ�����ټ�����ȴ������ֱ���������γ����IJ����0.001gΪֹ������Ϊ��ȷ����Ʒ�нᾧˮ�Ƿ��Ѿ���ȫ����ȥ��
��6���ڲⶨ����������Ʒ�к��м��Ȼӷ������ʻ�ʵ��ǰ������Ʒ���泱ʪ��������ɲ������ƫ�ߣ�
��1��������ƽ��ȷ��Ϊ0.1g��������ƽ�ɾ�ȷ��0.001g���������ݿ�֪��Ӧѡ�������ƽ��
�ʴ�Ϊ��������ƽ��
��3��������ˮ����ͭ���ˮ����Ӧ���ڸ��������ܷ���ȴ��
�ʴ�Ϊ����������
��4��Ϊȷ������ͭ������ȫʧȥ�ᾧˮ���������ȴ�����ټ�����ȴ������ֱ���������γ����IJ����0.001gΪֹ������Ϊ��ȷ����Ʒ�нᾧˮ�Ƿ��Ѿ���ȫ����ȥ����֤�ᾧˮȫ��ʧȥ��
�ʴ�Ϊ��ȷ���ᾧˮʧȥ��ȫ ��0.001��
��5�� ![]() = CuSO4+ xH20
= CuSO4+ xH20
160+18x 160
(24.692-13.721) (20.631-13.721)
![]() =
=![]()
��ã�x=5.22
ʵ��������=![]() ��100%=4.4%��
��100%=4.4%��
�ʴ�Ϊ��5.22��4.4%��
��6��a.������Ʒ�к��м��Ȳ��ӷ������ʻᵼ�²ⶨ��ˮ������ƫС����a����
b.�ڼ��ȹ����з����к�ɫ�������ɣ�˵������ͭ�ֽ⣬ʹ����ͭ������ƫС���ᾧˮ������ƫ���½��ƫ��b��ȷ��
c.���ȹ��������������彦��������ˮ�������ⶨ���ƫ��c��ȷ��
d.���Ⱥ�����δ�������������ȴ���ᵼ�²ⶨ������ͭ������ƫ�ⶨ��ˮ������ƫС����d����
�ʴ�Ϊ��bc��


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ��зḻ�ĺ�ˮ��Դ����ˮ�е�Ԫ�ع�����80���֣��ܴ����ܴ���Ԫ�ذ���H��B��C��O��F��Na��Mg��S��Cl��K��Ca��Br��Sr��13��Ԫ�أ�ͬʱ������Cr��Mn��Fe��Ni��Cu��Zn����Ԫ�أ���˿������ú�ˮ��Դ���зdz�������ǰ����
��1�������漰����Ԫ���У�������ǰ�����ڵ���___����̬ԭ���е�δ�ɶԵ����������___��дԪ�ط��ţ���
��2����̬Fe3+�ļ۵����Ų�ʽ___����̬Bԭ�ӵĵ����Ų�ͼΪ___��
��3��B��C��O��F����Ԫ�ػ�̬ԭ�ӵ�һ�������ɴ�С��˳��Ϊ___��дԪ�ط��ţ���
��4��CO32-������ԭ�Ӽ۲���Ӷ���Ϊ___�ԣ�SO32-�Ŀռ乹��Ϊ___��HCHO��C���ӻ���ʽΪ___��
��5��������������ˮ����Ҫԭ��֮һΪNH3��H2O����֮������γ��������ˮ�д��ڵ������___�֡�
��6���ɱ���ˮ���ۻ����ƻ�����Ҫ���÷ֱ���___��___��
��7����֪�Ƶ��ܶ�Ϊag/cm3��NAΪ�����ӵ�������ֵ���Ƶľ����ṹ��ͼ�����ⳤΪ___ pm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƾ���(Na2S2O3��5H2O)���������մ���֪��������ˮ���������Ҵ��������Ի���Ի������ȶ������ȡ������ֽ⡣ijʵ����ģ�ҵ���ȡ��������ƣ��䷴Ӧװ�ü������Լ���ͼ��
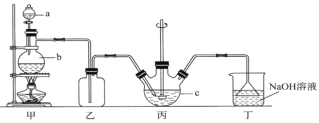
��1��װ�ü��У�a������������____________��a��ʢ��Ũ���ᣬb��ʢ���������ƣ�ʵ����Ҫ����SO2�������ʣ����Բ�ȡ�Ĵ�ʩ��_________________________(д��һ������)��
��2��װ���ҵ�������____________________________________��
��3��װ�ñ��У���Na2S��Na2CO3��2��1�����ʵ���֮�������Һ��ͨ��SO2������Ƶ�Na2S2O3��CO2����Ӧ�Ļ�ѧ����ʽΪ��________________________________________��
��4����ʵ�����õ�Na2CO3�к�����NaOH�����麬��NaOH��ʵ�鷽��Ϊ_________��(ʵ���й�ѡ�õ��Լ���������CaCl2��Һ��Ca(OH)2��Һ����̪��Һ������ˮ��pH�ơ��ձ����Թܡ��ιܣ���ʾ������ʱCaCO3������Һ��pH=9.5)
��5����Ӧ��������˱��еĻ�����Һ���������ᾧ�����ˡ�ϴ�ӡ�����ȵõ���Ʒ�����ɵ���������ƴ�Ʒ����_____________ϴ�ӡ�Ϊ�˲ⶨ�ֲ�Ʒ��Na2S2O3��5H2O�ĺ�����������������������KMnO4��Һ�ζ��ķ���(����ֲ�Ʒ�е�����������KMnO4��Һ����Ӧ)����ȡ1.50g�ֲ�Ʒ����ˮ����0.20 mol��L��1KMnO4��Һ(������ϡ�����ữ)�ζ�������Һ��![]() ȫ��������Ϊ
ȫ��������Ϊ![]() ʱ�����ĸ��������Һ���40.00mL��
ʱ�����ĸ��������Һ���40.00mL��
��д����Ӧ�����ӷ���ʽ��________________________________________��
�ڲ�Ʒ��Na2S2O3��5H2O����������Ϊ____________________(����С�����һλ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ�隣������̿��Ʊ��ߴ���̼���̵Ĺ����������£�
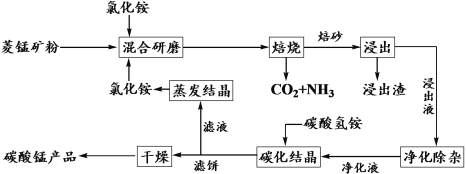
��֪�������̿�ʯ��Ҫ�ɷ���MnCO3������������Fe��Al��Ca��Mg��Ԫ�أ�
����ؽ�������[c(Mn+)=0.1 mol��L1]�γ��������������pH���£�
�������� | Al3+ | Fe3+ | Fe2+ | Ca2+ | Mn2+ | Mg2+ |
��ʼ������pH | 3.8 | 1.5 | 6.3 | 10.6 | 8.8 | 9.6 |
������ȫ��pH | 5.2 | 2.8 | 8.3 | 12.6 | 10.8 | 11.6 |
�ش��������⣺
(1)�����ա�ʱ��Ҫ��Ӧ�Ļ�ѧ����ʽΪ__________________��
(2)��������ͼ1��ͼ2��ͼ3���Ȼ�隣�����þ�����������ǣ�_________________��

(3)����Һ���������ӡ��������£����ȼ���MnO2��Fe2+����ΪFe3+����Ӧ�����ӷ���ʽΪ________________________��Ȼ�������ҺpHʹFe3+��Al3+������ȫ��
(4)̼���ᾧʱ��������Ӧ�����ӷ���ʽΪ___________��̼���ᾧ�����в�����̼�����Һ����̼�������Һ���ܵ�ԭ����___________________��
(5)��������ѭ�����õĹ�̬������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳɸ߷��ӽ����������ϼ��ķ�Ӧ��
 +(2n-1)H2O
+(2n-1)H2O
����˵������ȷ����
A.�ۺ���Ӧ������������ʣ���NaOH�����ڽӴ�
B.�ۺ���ĺϳɷ�ӦΪ���۷�Ӧ
C.1mol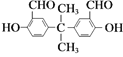 ������������Һ���ÿ�����2mol��
������������Һ���ÿ�����2mol��
D.ͨ�������ⶨ��ƽ����Է����������ɵ���ۺ϶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���HA��NaA�Ļ����Һ�� c(HA)��c(A��)=0.1 mol��L��1����Һ��c(HA) ��c(A��)�Ĵ�С����pH�仯�Ĺ�ϵ��ͼ��ʾ�������й���������ȷ����
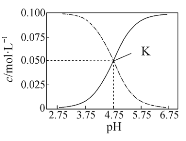
A.�����£�HA�ĵ���ƽ�ⳣ��ԼΪ10��5
B.��pH=3.75��Һ�У�c(Na��)��c(H��)��c(HA) = c(OH��) +0.1 mol��L��1
C.��pH=5.75��Һ�У�c(OH��)��c(H��)��c(A��)��c(HA)
D.��K�����Һ�У�HA�ĵ���̶ȴ���A����ˮ��̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ͬ����Ԫ�أ��䵥�ʺͻ�������������������;�ܹ㡣
SO2�����ڷ���������������Ҳ��һ����Ҫ���䶳���ʡ�ʵ���ҿ�����ͼ��ʾװ���Ʊ�SO2�����ô���SO2�������ʵ�顣
(1)���������Ʊ���SO2���������������СҺ�ζ��ʰ���״����ȥ���������Ʊ�װ�ú����ӳ���װ�ã��뻭������װ�ò�����װ���е��Լ�___________��
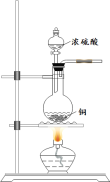

(2)��SO2ͨ��0.1mol/L Ba(NO3)2��Һ�õ���ɫ�������÷�Ӧ�����ӷ���ʽΪ_______��
�ֱ�����к�δ��й�������ˮ����Ba(NO3)2��BaCl2��Һ����������ʵ�飺
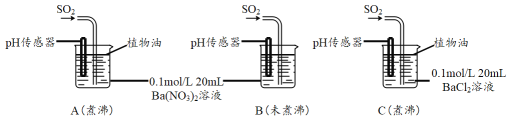
(3)ʵ��C�У�û�й۲쵽��ɫ��������pH��������ʾ��Һ�����ԣ��û�ѧ�����ʾ��ԭ��______________________________��
(4)ʵ��B�г��ְ�ɫ������ʵ��A��ܶࡣ�ɴ˵ó��Ľ�����_________________��
(5)�ⶨˮ���ܽ�O2���������õķ����ǣ�
i����ȡa mLˮ����Ѹ�ټ�������MnSO4��Һ������NaOH��KI��Һ�������������ӣ���ʹ��Ӧ���ȡ�
ii��������Ѹ�ټ������������ᣬ��ʱ��I2���ɡ�
iii����ii������Һ�еμ�2�ε�����ҺΪָʾ������b mol/LNa2S2O3����Һ�ζ����յ㹲������Na2S2O3��ҺV mL��
�йط�Ӧ����ʽΪ��2Mn2+ + O2+ 4OH- = 2MnO(OH)2����Ӧ�ܿ죩
MnO(OH)2 + 2I- + 4H+= Mn2+ + I2 + 3H2O
I2 + 2S2O32- = 2I- + S4O62-
��ˮ���ܽ� O2��������mg/LΪ��λ��Ϊ___________________��
���жϴﵽ�ζ��յ��ʵ������Ϊ __________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������벻����ѧ���磺����������Ƭ��������ƶѪ(��ͼ����˵����)���ڸ�������������������θ����࣬������������ˮ�����ȵȡ�

�Ķ��Ϸ�����������Ƭ���ı�ǩ˵�����ش�
��1�� �������У�����ϡ����1�Ρ���������_____________________________________�����Ǽ�ϡ�����ԭ����_______________________________________��
��2������������������θ�����ʱ������������Ϊ_________�к���θ���������Ҳ�ܸ��ռ���Һ��Ӧ����Ӧ�����ӷ���ʽΪ_________________________��
��3�������ܾ�ˮ����Ϊ��Al3+��ˮ�⣬��ˮ�����������������____________���������һ����ʵ����֤��������ˮ������ˮ��__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����9.2g������Ͷ��������ˮ��D2O���У�������������к��У� ��
A.0.2 mol����B.0.2 mol����C.0.4 mol����D.0.4 mol����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com