
����Ŀ�����������벻����ѧ���磺����������Ƭ��������ƶѪ(��ͼ����˵����)���ڸ�������������������θ����࣬������������ˮ�����ȵȡ�

�Ķ��Ϸ�����������Ƭ���ı�ǩ˵�����ش�
��1�� �������У�����ϡ����1�Ρ���������_____________________________________�����Ǽ�ϡ�����ԭ����_______________________________________��
��2������������������θ�����ʱ������������Ϊ_________�к���θ���������Ҳ�ܸ��ռ���Һ��Ӧ����Ӧ�����ӷ���ʽΪ_________________________��
��3�������ܾ�ˮ����Ϊ��Al3+��ˮ�⣬��ˮ�����������������____________���������һ����ʵ����֤��������ˮ������ˮ��__________________________________��
���𰸡�����FeSO4ˮ�� ��ϡ����������������ӵļ��� ������ Al(OH)3+OH��=AlO2��+2H2O ���� ��pH��ֽ��pH�Ʋ���������Һ��pH < 7 ֤����Һ�����ԣ�����ʯ����Һ����������Ҳ���ԣ�
��������
��1����������Ϊǿ�������Σ�����ˮ����ˮ�⣬�Ӷ�ʹ��Һ����ǣ���������������Ƭ�ܽ�����У�����������ˮ�⣬�ں�����֤�У�����Ҫ��֤��������ӣ��Ӽ������ữ������������������ӣ�����ԭҩƬ����������ӵļ��飬�ʴ�Ϊ������FeSO4ˮ�⣻��ϡ����������������ӵļ��飻
��2��θ��Ϊ���ᣬ�����������к��ᣬ�Ӷ����ֳ����ԣ������������ռNaOH����Һ��Ӧ�����ӷ�Ӧ����ʽΪ��Al(OH)3+OH��=
AlO2��+2H2O���ʴ�Ϊ���Al(OH)3+OH��=AlO2��+2H2O��
��3�������ܾ�ˮ����ΪAl3+��ˮ������Al(OH)3�Ľ��壬��������������������ˮ�е��������pH��ֽ��pH�Ʋ���������Һ��pH < 7 ֤����Һ�����ԣ�����ʯ����Һ����������Ҳ���ԣ����ʴ�Ϊ����������pH��ֽ��pH�Ʋ���������Һ��pH < 7 ֤����Һ�����ԣ�����ʯ����Һ����������Ҳ���ԣ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��a��b��c��d��ԭ��������������������ת����ϵ�У��ס��ҡ�����������Ϊ��������Ԫ����ɵĶ�Ԫ����Ԫ���������AΪdԪ����ɵĵ��ʣ���������ΪҺ�壬�����ʳ�����������Ư�ס�����˵���������
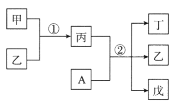
A.�����Ӱ뾶��c��b
B.���м������Ӽ����м��Լ�
C.b��c�γɵĻ�������������������Ŀ��Ϊ1��2
D.a��b��d�γɵĻ������У�d���ӻ���ʽ��sp3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��������ͭ����(![]() )�ᾧˮ�����IJⶨʵ�顣���������գ�
)�ᾧˮ�����IJⶨʵ�顣���������գ�
��ʵ�鲽�裩��
(1)��__________(����������)ȷ������������������
(2)�ڴ������м���һ����������ͭ���壬�����ء�
(3)��ʢ������ͭ����Ĵ������������������������ȣ�ֱ����ɫ��ȫ��ף�Ȼ�����������_______(����������)����ȴ�����£������ء�
(4)�ظ�����ʵ����к��ز�������Ŀ����_______________��ֱ�����γ������������______�ˡ�
(5)�����Ǹ�ѧ��ʵ���һ�����ݣ�����ɼ��㣺
��������(��) | �����뾧�������(��) | ���غ�������������� |
13.721 | 24.692 | 20.631 |
![]() ______��(��ȷ��0.01)ʵ����������_________(����С����һλ)��
______��(��ȷ��0.01)ʵ����������_________(����С����һλ)��
(6)���ʵ���в�������ԭ�������_______
a. ����ͭ�����к��в��ӷ������� b. �ڼ��ȹ����з����к�ɫ��������
c. ����ʱ�о���ɽ����� d. ����ʧˮ��¶���ڿ�������ȴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������M��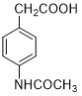 ����һ�ֿ���ʪ�Թؽ���ҩ���ϳ�·�����£�
����һ�ֿ���ʪ�Թؽ���ҩ���ϳ�·�����£�

��1����Ӧ1������Ϊ______________________________��
��2����Ӧ2���Լ�Ϊ______________________________��
��3��д��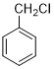 �����к������ṹ��ͬ���칹��Ľṹ��ʽ______________��
�����к������ṹ��ͬ���칹��Ľṹ��ʽ______________��
��4��д���ɶ����������ᣨ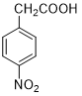 ���õ��������������������Լ�������______��
���õ��������������������Լ�������______��
��5��A�ķ���ʽΪC7H8��д����A�ϳɱ���ȩ�ĺϳ�·�ߡ�
���ϳ�·�߳��õı�ʾ��ʽΪ�� ��
��
_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ȼ�ǵ�����ȵ����⡣�о�������������Ҫ���塣�ش��������⣺
��1��2NO(g)+O2(g)![]() 2NO2(g)�ķ�Ӧ���̷�������
2NO2(g)�ķ�Ӧ���̷�������
��2NO(g)![]() N2O2(g) �� ��H1<0
N2O2(g) �� ��H1<0
��N2O2(g)+O2(g)![]() 2NO2(g) �� ��H2<0
2NO2(g) �� ��H2<0
��Ӧ2NO(g)��O2(g)![]() 2NO2(g)��H=__(�ú�H1��H2��ʽ�ӱ�ʾ)����Ӧ�ٵĻ��E1�뷴Ӧ�ڵĻ��E2�Ĵ�С��ϵΪE1__E2(����>������<������=��)��
2NO2(g)��H=__(�ú�H1��H2��ʽ�ӱ�ʾ)����Ӧ�ٵĻ��E1�뷴Ӧ�ڵĻ��E2�Ĵ�С��ϵΪE1__E2(����>������<������=��)��
��2����ҵ�ϳ�����CH4������CO2����CO��H2������H2��CO�ϳɼ״������ں����ܱ�������ͨ�����ʵ���Ũ�Ⱦ�Ϊ1.0mol��L-1��CH4��CO2����һ�������½�������Ӧ��CO2(g)+CH4(g)![]() 2CO(g)+2H2(g)�����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ1��ʾ��
2CO(g)+2H2(g)�����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ1��ʾ��
�ٸ÷�Ӧ����H___(����<����=������>��)0��
��ѹǿp1��p2��p3��p4�ɴ�С�Ĺ�ϵΪ___���жϵ�������______��ѹǿΪp4ʱ����b�㣺v(��)___(����<����=������>��)v(��)��
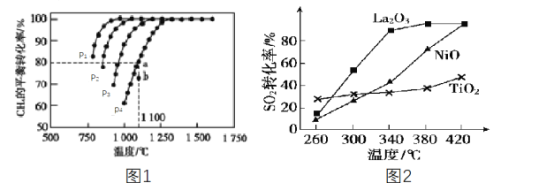
�۶������෴Ӧ����ij���(B)��ƽ��ѹǿp(B)�������ʵ���Ũ��c(B)Ҳ�ɱ�ʾƽ�ⳣ��(����Kp)����p4=0.36MPa����a���ƽ�ⳣ��Kp=___(����3λ��Ч���֣���ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��
��3��ȼú��������Ļ��շ�ӦΪ2CO(g)��SO2(g)=2CO2(g)��S(l)��������������ͬ��������ͬʱ����Ļ��շ�Ӧ��SO2��ת�����淴Ӧ�¶ȵı仯��ͼ2��ʾ��260��ʱ��____ (����La2O3��NiO������TiO2��)�Ĵ�Ч����ߡ�La2O3��NiO������������ʹSO2��ת���ʴﵽ�ܸߣ������Ǽ۸����أ�ѡ��La2O3����Ҫ�ŵ���__��
��4����С����Na2SO3��Һ�������SO2�õ�NaHSO3��Һ��Ȼ�������Һ�Ƶ������ᡣԭ����ͼ3��д����ʼ���ʱ�����ĵ缫��Ӧʽ__��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ⶨ����ͭ���壨CuSO4XH2O ����Xֵ��ʵ��������£�
![]()
��1��������ʵ�����õ��ļ����������������Ӧ������ȷ����___________��
��2�������ա�ʱ��Դѡ�õ��Ǿƾ��ƶ����Ǿƾ���ƣ�������_____________________������ȴ������_______________��(����������)��
��3�� �����ء�������Ŀ����_________________________________________________��
�жϡ����ء���������_________________________________________________��
��4��������ijѧ��ʵ���һ�����ݣ�����ɼ���
�������� | �����뾧�������� | ���Ⱥ���������������� |
11.721g | 22.692g | 18.631g |
X=__________________��(��ȷ��0.01)��ʵ����������_________________��(����С�����һλ)
��5�����ʵ���в�������ԭ�������__________����ɵġ�
a������ͭ�����к��в��ӷ������ʡ��� b���ڼ��ȹ��̷����к�ɫ��������
c������ʱ�о���ɽ����������������� d������ʧˮ��¶���ڿ�������ȴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܲ����أ��ṹ��ͼ���Ǿ��п���������Ч������Ȼ����֮һ����һЩʮ�ֻ���ֲ���к����Ϸḻ�������������ֶ�����Ԫ�ع��ɣ�����W��X��Y��Z��ԭ��������������Y��Zԭ�Ӻ���������������ȡ���������һ����ȷ���ǣ� ��
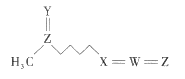
A.ԭ�Ӱ뾶�Ĵ�С˳��ΪZ>W>X>Y
B.X�ļ��⻯����W���⻯�ﷴӦ�������ӻ�����
C.�ܲ������еĸ�Ԫ��ԭ������������8�����ȶ��ṹ
D.Y��Z�γɵĶ�Ԫ�������ˮ����Ϊǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���ѧ�Լ������裨��ͼ��ʾ���������Ʊ��ǰ���ҩ�����ҩ�ȡ������йذ�����˵����ȷ���ǣ� ��

A.���ڲ�������B.����������ԭ�ӹ���
C.ˮ���ԽϺ�D.�ܷ���ȡ�����ӳɡ���ȥ�������ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������25��ʱ0.1molL-1�İ�ˮ����ش��������⣺
��1����ˮ�ʼ��Ե�ԭ��Ϊ�������ӷ���ʽ��ʾ��___��
��2������ˮ�м���ϡ���ᣬʹ��ǡ���кͣ�д����Ӧ�����ӷ���ʽ___����������Һ��pH=5������Һ��ˮ���������������Ũ��Ϊ___��
��3������ˮ�м�����������粒��壬��ʱ��Һ��![]() __������������������С����������������
__����������������������������������
��4������ˮ�м���ϡ��������Һ��pH=7����ʱc��NH4+��=amolL-1����c��SO42-��=__��
��5������ˮ�м���pH=1�����ᣬ�Ұ�ˮ������������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����___��
��6����������ͨ��0.1mol/L���Ȼ�����Һ�������ͣ����������������백ˮ��������ɫ�������õ���ƽ�����ԭ��___��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com