
����Ŀ���Ȼ��ƣ�NaCl���������г��õĻ�ѧƷ��Ҳ����Ҫ�Ļ�������ԭ�ϡ�
��1��ijͬѧ������Ԫ�صIJ�ͬ��������Ʊ�NaCl�������о�3�ַ�Ӧ��
��2Na+Cl2![]() 2NaCl
2NaCl
��___��
��___��
��___��
��2��������һ�㺬��CaCl2��MgCl2��CaSO4��MgSO4���ʡ��Ӵ����Ƶþ��εĹ������£�

��д��MgCl2��CaSO4�ĵ��뷽��ʽ��___��___��
�ڸ������ֱ��ǣ�����a___������b___������c___��
���Լ�����___��
�ܼ����Լ��������������ӷ���ʽΪ___�������Լ��������������ӷ���ʽΪ___��
���𰸡�Na2O+2HCl=2NaCl+H2O NaOH+HCl=NaCl+H2O Na2CO3+2HCl=2NaCl+H2O+CO2�� MgCl2=Mg2++2Cl- CaSO4=Ca2++SO42- ���� ���� �����ᾧ��Һ BaCl2 CO32-+Ba2+=BaCO3����CO32-+Ca2+=CaCO3�� CO32-+2H+=H2O+CO2����OH-+H+=H2O
��������
��1����Na2O�����ᷴӦ�������Ȼ��ƣ�
������кͷ�Ӧ�ܹ������Ȼ��ƣ�
�ܴ��������ᷴӦ�������Ȼ��ƣ�
��2�������д��ڲ�������ɳ�Ϳ����Ե�CaCl2��MgCl2��CaSO4��MgSO4�����ʣ����˿ɳ�ȥ��������ɳ������BaCl2��NaOH��Na2CO3�ɽ�����һһ��ȥ���ݴ˻ش����⡣
��1����Na2O�����ᷴӦ�������Ȼ��ƣ���ӦΪ��Na2O+2HCl=2NaCl+H2O��
������кͷ�Ӧ�ܹ������Ȼ��ƣ���ӦΪ��NaOH+HCl=NaCl+H2O
�ܴ��������ᷴӦ�������Ȼ��ƣ���ӦΪ��Na2CO3+2HCl=2NaCl+H2O+CO2����
��2�������д��ڲ�������ɳ�Ϳ����Ե�CaCl2��MgCl2��CaSO4��MgSO4�����ʣ����˿ɳ�ȥ��������ɳ��������aΪ���ˣ����õ���ҺA�д���CaCl2��MgCl2��CaSO4��MgSO4�����ʣ����������BaCl2��Һ���ɳ�ȥSO42-�����������NaOH��Һ��ȥ���е�Mg2+�����������Na2CO3�ɳ�ȥ��Һ�����е�Ba2+�������г���һ���Թ��˺����ҺB����ô����bΪ���ˣ��Լ�����BaCl2��Һ���Լ�����Na2CO3��Һ����ҺB�д���NaCl������Na2CO3��Һ��������������Ტ���ȣ��ɳ�ȥ������Na2CO3��Һ�����յõ�������NaCl��Һ�����Լ���Ϊ���ᣬ����cΪ�����ᾧ��
��MgCl2�ĵ��뷽��ʽΪ��MgCl2=Mg2++2Cl-��CaSO4�ĵ��뷽��ʽΪ��CaSO4=Ca2++SO42-��
���ɷ�����֪������a�ǹ��ˣ�����b�ǹ��ˣ�����c�������ᾧ��
���ɷ�����֪���Լ�����BaCl2��Һ��
�ܼ����Լ��������������ӷ���ʽΪCO32-+Ca2+=CaCO3����CO32-+Ba2+=BaCO3���������Լ��������������ӷ���ʽΪCO32-+2H+=H2O+CO2����OH-+H+=H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ȣ�![]() ��������Ϊ��ɫҺ�壬�۵�-54.1�棬�е�69.l�棬100�����ϻ�ʱ�����ֽ⣬�ڳ�ʪ�����С����̡����������л���ѧ���Ȼ�������ҩ���Ⱦ�ϵ���ȡ��Ҳ����Ҫ���á�ʵ�����п��ø���������Ͷ��������ڻ���̿������ȡ�����ȣ�
��������Ϊ��ɫҺ�壬�۵�-54.1�棬�е�69.l�棬100�����ϻ�ʱ�����ֽ⣬�ڳ�ʪ�����С����̡����������л���ѧ���Ȼ�������ҩ���Ⱦ�ϵ���ȡ��Ҳ����Ҫ���á�ʵ�����п��ø���������Ͷ��������ڻ���̿������ȡ�����ȣ�![]()
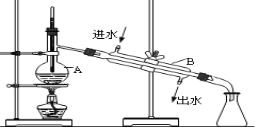
![]() ��ʵ��װ����ͼ��ʾ�����ּг�װ��δ��������
��ʵ��װ����ͼ��ʾ�����ּг�װ��δ��������
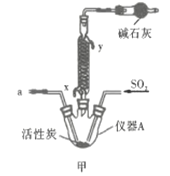
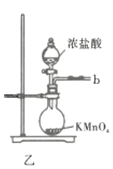
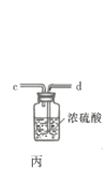
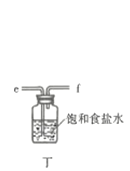
�ش��������⣺
��1������A������Ϊ___________�����������������ҵķ�������װ�ýӿ�����˳��Ϊ___________��
��2������������������ȴˮ�������___________���x����y�������������ʢװ��ʯ�ҵ�����Ϊ___________��
��3�����ڴ�ŵ������Ȼᷢ�ƣ���ԭ�����Ϊ______________________��
��4��![]() �ڳ�ʪ�����С����̡�����Ӧ�Ļ�ѧ����ʽΪ______________________��
�ڳ�ʪ�����С����̡�����Ӧ�Ļ�ѧ����ʽΪ______________________��
��5����ҵ���Ƶõ������ȳ��������������ʣ����õζ����ⶨ�䴿�ȣ���ȡ1.500g��Ʒ�����뵽ʢ��100mL 0.5000 mol![]() ��Һ���ձ��м��ȳ�ַ�Ӧ����ȴ��ת����250mL___________�У�����___________����ҡ�����ݡ�ҡ�ȣ����Ƶõ�������Һ��ȡ25.00mL������Һ����ƿ�У��μ�2�μ��ȣ���0.1000
��Һ���ձ��м��ȳ�ַ�Ӧ����ȴ��ת����250mL___________�У�����___________����ҡ�����ݡ�ҡ�ȣ����Ƶõ�������Һ��ȡ25.00mL������Һ����ƿ�У��μ�2�μ��ȣ���0.1000![]() ����Һ�ζ�������ζ��յ������Ϊ___________���ظ��ζ����Σ�ƽ�����ı���Һ10.00mL����ø���Ʒ�Ĵ���Ϊ___________��
����Һ�ζ�������ζ��յ������Ϊ___________���ظ��ζ����Σ�ƽ�����ı���Һ10.00mL����ø���Ʒ�Ĵ���Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪʵ������������ˮ�õ���������ˮ��װ��ʾ��ͼ�������ͼʾ�ش��������⣺
��1��װ��������A��B��������_____________________ �� ______________________��
��2����ָ����ͼ�е��������Դ���____________________��_____________________��
��3��ʵ��ʱA�г�������������ˮ�⣬������������ķ�ʯ����������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������NH3����һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ�����Ƶ��ʡ���ҩ���ϳ���ά�ȡ���ش��������⣺
��1��2molNH3�ڱ�״���µ������___��
��2��2molNH3��������___������������Ԫ�ص�������___��
��3��2molNH3�����İ�������Ϊ___��������Ϊ___��
��4����ҵ���÷�ӦN2+3H2 ![]() 2NH3���ϳɰ����������Ƶ�2molNH3����״���²��뷴Ӧ��H2�����Ϊ___��14��N2���뷴Ӧ�ɵ�NH3���ʵ���Ϊ___��
2NH3���ϳɰ����������Ƶ�2molNH3����״���²��뷴Ӧ��H2�����Ϊ___��14��N2���뷴Ӧ�ɵ�NH3���ʵ���Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��������Ĺ�������ʾ��ͼ���£�

���������գ�
��1������ˮ���������A��B�����ʣ�������A��Դ��ʯ��Ҥ������д��A��B�Ļ�ѧʽ��
A__________________________________B__________________________________
��2��ʵ�����ᴿ���ε�ʵ���������Ϊ��
ȡ����______��������______��______����ȴ�ᾧ��______����ɡ�
��3����ҵ��������������У�̼�ữʱ������������____________________________��
̼�ữʱû������̼���ƾ��壬��ԭ����_______________________________________��
��4��̼�ữ����ˣ���ҺD����Ҫ�ijɷ���_____________________________����д��ѧʽ����������һ�ɷֵ������ӵľ��巽���ǣ�________________________________________��
��5����������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ��ˮ����������ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ��_________________________________________________________
��ҺD��ʯ��ˮǰ��Ҫ���ȣ�ԭ����_____________________________________________��
��6����Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ��
_____________________________________________________________________________
��ע����ı���ʽ�����õ��йط��ŵĺ��壩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ں��¡��ݻ�������ܱ������н������·�Ӧ��N2(g)��3H2(g)![]() 2NH3(g)���ﵽƽ�⡣����˵������ȷ���ǣ� ��
2NH3(g)���ﵽƽ�⡣����˵������ȷ���ǣ� ��
A. �����������䣬��С���������ƽ�������ƶ���Kֵ���
B. �����������䣬ͨ������������ƽ�ⲻ�ƶ�
C. N2��H2��NH3�ٷֺ������ٱ仯
D. �����������䣬�����¶ȣ���ѧƽ��һ�������ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ���Ҳ�����ȷ���ǣ� ��
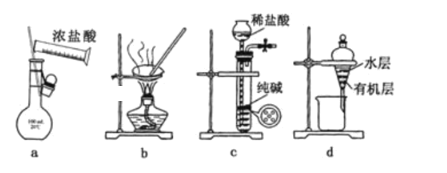
A.��ͼa��ʾװ������l00mL0.100mo1L-1ϡ����
B.��ͼb��ʾװ������NaCl��Һ�Ʊ�NaCl����
C.��ͼc��ʾװ����ȡ����CO2����
D.��ͼd��ʾװ�÷����ñ���ȡ��ˮ���ѷֲ���л����ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������ԭ��Ϊ0.2��6.02��1024����NH4HCO3����������___�����к���ԭ�ӵ����ʵ���Ϊ___��3.6��ˮ�к���������Ϊ___������٤������ΪNA����
��2����ʵ����Ҫ��12mol��L-1����������Һ������Ũ��Ϊ0.6mol��L-1��ϡ����������Һ100mL������Ҫ����Ũ��������___mL����Ҫ��������ƽ����___���������Ʋ������Ƴ�0.5mol��L-1������������Һ100mL��
��3���������aLHCl�����������Ƴ�1L������Һ��������Һ�ܶ�Ϊdg/cm3����������Һ���ʵ���Ũ��Ϊ___mol/L������Һ�����ʵ���������Ϊ___��
��4���ڱ�״���£�ij����A���ܶ���1.25g��L-1��������Ħ��������___��ͬ��ͬѹ�¸�����������������ܶ���___���ڱ�״���£���CO��CO2��ɵĻ������Ϊ6.72L������Ϊ12g���˻�������ƽ����Է���������___���������CO��CO2���ʵ���֮����___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijһ��Ӧ��ϵ�е�������HCl��SnCl2��H2SnCl6��As��H3AsO3��H2O����֪��HCl�Ƿ�Ӧ��֮һ��
(1)��д��δ��ƽ�ĸ÷�Ӧ�Ļ�ѧ����ʽ��____��___
���ڸ÷�Ӧ��,�õ��ӵ�������___����������Ԫ����___��
���ڷ�Ӧ�У�ÿת��1 mol���ӣ�����HCl___mol��
��������������ȷ����___(��д��ĸ���)��
a.����ͬ�����£�����A��Ԫ�ص������ӵĻ�ԭ�Դ��ϵ�������ǿ
b.�õ���Խ���������,��������Խǿ
c.������ֻ����������
d.����ͬ�����£�������˳��Fe3+>Cu2+>H+>Fe2+
(2)����ʢ��KI��Һ���Թ��м�������CCl4��μ���ˮ��CCl4������ɫ���÷�Ӧ�����ӷ���ʽΪ__������������Թ��еμ���ˮ����CCl4�����dz,�������ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ___��
����KI����KBr����CCl4���Ϊ____ɫ;�����μ���ˮ��CCl4�����ɫû�б仯��Cl2��HIO3��HBrO3��������ǿ������˳����____��
���ӵ����к�����Ϊ20mg��kg-1��50 mg��kg-1����ȡ�ӵ���(��KIO3��ʳ��)1000kg������KI��Cl2��Ӧ��KIO3��������Ҫ����Cl2____L(��״��������2λС��)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com