
�����й�Ԫ�����ʵ�˵������ȷ����
A���������е����Ų�ʽ��ԭ���У���1s22s22p63s23p2����1s22s22p3����1s22s22p2����1s22s22p63s23p4��ԭ�Ӱ뾶�����Ǣ�
B���������м۵����Ų�ʽ��ԭ���У���3s23p1����3s23p2����3s23p3����3s23p4����һ�����������Ǣ�
C����Na��K��Rb����O��S��Se����Na��P��Cl��Ԫ�صĵ縺����ԭ������������������Ǣ�
D��ijԪ����̬��̬ԭ�ӵ������ֱܷ�Ϊ738��1 451��7 733��10 540��13 630��17 995��21 703��������������Ӧʱ�������ɵ���������X3��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2008����ҹ��Ϸ������ı�ѩ�ֺ��У�ʹ����һ����ѩ��������Ҫ�ɷֵĻ�ѧʽΪXY2��X��Y��Ϊ���ڱ�ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1 mol XY2����54 mol���ӡ�
��1������ѩ���Ļ�ѧʽ��__________��X����Ԫ���γɵĻ�����ĵ���ʽ��_____________��
��2��Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D��Y���ڣ���D�����ӽṹʾ��ͼ��________________��D��E���γ�һ�ַǼ��Է��ӣ��÷��ӵĽṹʽΪ________________��D������Ԫ�ص��⻯���У��е���͵���_________________________��
��3��Ԫ��W��Yͬ���ڣ��䵥����ԭ�Ӿ��壻Ԫ��Z�ĵ��ʷ���Z2����3�����ۼ���W��Z���γ�һ���������ǽ������ϣ��仯ѧʽ��____________________________________��
��4��Ԫ��R��Yͬ���壬���⻯�������ڿ�ʴ������R2��NaOH��Һ��Ӧ�IJ���֮һ��OR2���÷�Ӧ�����ӷ���ʽΪ______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ��X��Y��ԭ���������2�������й�������ȷ����
A.X��Y������λ��ͬһ����
B.X��Yһ��λ��ͬһ����
C.X��Y�����γɹ��ۻ�����XY
D.X��Y�����γ����ӻ�����XY
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D��E���ֶ�����Ԫ�أ�����A��B��C����ͬһ���ڣ�Aԭ�������p�ܼ��ϵĵ��������ڴ����ĵ���������Bԭ���������������δ�ɶԵĵ��ӣ�D��Eԭ�Ӻ��ڸ��Ե�����������������ȣ�BԪ�ؿɷֱ���A��C��D��EԪ���γ�RB2�ͻ��������DB2��EB2�У�D��B������֮��Ϊ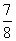 ��E��B������֮��Ϊ1�����������������ش��������⣺
��E��B������֮��Ϊ1�����������������ش��������⣺
(1)�ƶ�����Ԫ�طֱ���(��Ԫ�ط���)��A_________��B________��C________��D________��E________��
(2)д��Dԭ�ӵĵ����Ų�ʽ��______________________________________��
(3)ָ��EԪ����Ԫ�����ڱ��е�λ�ã�__________________________________��
(4)�Ƚ�A��B��C����Ԫ�ص�һ�����ܵĴ�С��_____________________________
__________________________________(дԪ�ط��Ż����ƣ���ͬ)��
(5)�Ƚ�Ԫ��D��E�ĵ縺�Ե���Դ�С��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ����±���
| Ԫ�ش��� | X | Y | Z | W |
| ԭ�Ӱ뾶/pm | 160 | 143 | 70 | 66 |
| ��Ҫ���ϼ� | ��2 | ��3 | ��5����3����3 | ��2 |
����������ȷ����
A��X��YԪ�صĽ�����X��Y
B��һ�������£�Z������W�ij�������ֱ������ZW2
C��Y������������Ӧ��ˮ����������ϡ��ˮ
D��һ�������£�W���ʿ��Խ�Z���ʴ����⻯�����û�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾװ����ϴ���������ȶ��ֹ��ܡ����й��ڸ�װ�õ���;��˵������ȷ����(����)
A����ȥO2�л��е�ˮ������ƿ��װŨ����(ƿ���ݻ���һ��)�������b�˽�
B������ˮ���ռ���������ƿ��װ��ˮ�������a�˽�
C�����ڲ���������ƿ��װˮ(ƿ���ݻ���һ��)��b�˽ӹ�����ƿ
D������������ˮ��������������ƿ��װ��ˮ�������b�˽���a�˽���Ͳ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�Ƿ�������ʱ���õ��������������ң����Խ��еĻ�����������ֱ���
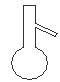
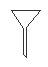
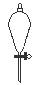
A��������������Һ������ B�������ˡ���Һ������
C����Һ�����ˡ��������� D�����ˡ���������Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں���4 mol HCl��1 mol MgSO4�Ļ����Һ����μ���Ba(OH)2��Һ�������ij���
| ����m�����Ba(OH)2���ʵ���n֮��Ĺ�ϵ����ͼ������˵����ȷ���� A��a��bʱ�ij����ijɷ�ΪBaSO4��Mg(OH)2 B��b��cʱ���������ӷ�ӦΪ��H+ + OH�� == H2O C��c��dʱ���ӵ����ʵ�����Ba2+ ���ܴ���Cl�� D��d��eʱ���ӵ����ʵ�����Ba2+ һ������OH�� |
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵ�鲻�ܴﵽԤ��Ŀ�ĵ���(����)
| ���ʵ�� | Ԥ��Ŀ�� | |
| A | ��ͬ�¶��£��������Ĵ���ʯ�顢����ʯ�۷ֱ�����������Ũ�ȵ����ᷴӦ | ̽���Ӵ�����Ի�ѧ��Ӧ���ʵ�Ӱ�� |
| B | ��װ����ɫ��ͬ��NO2��N2O4���������֧�Թ�(�ܷ�)�ֱ������ˮ����ˮ�� | ̽���¶ȶԻ�ѧƽ���Ӱ�� |
| C | �������м���ϡ���ᣬˮԡ���ȣ��ټ������Ƶ�������ͭ������ | ̽������ˮ�������л�ԭ�� |
| D | ��֧�Թ���װ�е��������Ũ�ȵ�H2O2��Һ��������һ֧�Թ��м���FeCl3��Һ | ̽��FeCl3��Һ��H2O2�ֽ����ʵ�Ӱ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com