
ЁОЬтФПЁПЧтФмЕФДцДЂЪЧЧтФмгІгУЕФжївЊЦПОБЃЌФПЧАЫљВЩгУЛђе§дкбаОПЕФжївЊДЂЧтВФСЯгаЃКХфЮЛЧтЛЏЮяЁЂИЛЧтдиЬхЛЏКЯЮяЁЂЬМжЪВФСЯЁЂН№ЪєЧтЛЏЮяЕШЁЃ
(1)Ti(BH4)2ЪЧвЛжжЙ§ЖЩдЊЫиХ№ЧтЛЏЮяДЂЧтВФСЯЁЃ
ЂйTi2+ЛљЬЌЕФЕчзгХХВМЪНПЩБэЪОЮЊ__________________ЁЃ
ЂкBH4-ЕФПеМфЙЙаЭЪЧ________________(гУЮФзжУшЪі)ЁЃ
(2)вКАБЪЧИЛЧтЮяжЪЃЌЪЧЧтФмЕФРэЯыдиЬхЃЌРћгУN2+3H2![]() 2NH3ЪЕЯжДЂЧтКЭЪфЧтЁЃ
2NH3ЪЕЯжДЂЧтКЭЪфЧтЁЃ
ЂйЩЯЪіЗНГЬЪНЩцМАЕФШ§жжЦјЬхШлЕугЩЕЭЕНИпЕФЫГађЪЧ__________________ЁЃ
ЂкЯТСаЫЕЗЈе§ШЗЕФЪЧ________(ЬюзжФИ)ЁЃ
a.NH3ЗжзгжаNдзгВЩгУsp3дгЛЏ
b.ЯрЭЌбЙЧПЪБЃЌNH3ЗаЕуБШPH3Ип
c.[Cu(NH3)4]2ЃЋжаЃЌNдзгЪЧХфЮЛдзг
d.CN-ЕФЕчзгЪНЮЊ![]()
(3)CaгыC60ЩњГЩЕФCa32C60ФмДѓСПЮќИНH2ЗжзгЁЃ
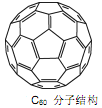
ЂйC60ОЇЬхвзШмгкБНЁЂCS2ЃЌЫЕУїC60ЪЧ________Зжзг(ЬюЁАМЋадЁБЛђЁАЗЧМЋадЁБ)ЃЛ
Ђк1ИіC60ЗжзгжаЃЌКЌгаІвМќЪ§ФПЮЊ________ИіЁЃ
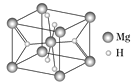
(4)MgH2ЪЧН№ЪєЧтЛЏЮяДЂЧтВФСЯЃЌЦфОЇАћНсЙЙШчЭМЫљЪОЃЌвбжЊИУОЇЬхЕФУмЖШЮЊa gЁЄcm-3ЃЌдђОЇАћЕФЬхЛ§ЮЊ____cm3[гУaЁЂNAБэЪО(NAБэЪОАЂЗќМгЕТТоГЃЪ§)]ЁЃ
ЁОД№АИЁП1s22s22p63s23p63d2(Лђ[Ar]3d2) е§ЫФУцЬх H2< N2< NH3 abcd ЗЧМЋад 90 ![]()
ЁОНтЮіЁП
(1)ЂйTiЪЧ22КХдЊЫиЃЌTiдзгЪЇШЅзюЭтВу2ИіЕчзгаЮГЩTi2+ЃЌШЛКѓИљОнЙЙдьдРэЪщаДЛљЬЌЕФЕчзгХХВМЪНЃЛ
ЂкИљОнМлВуЕчзгЖдЛЅГтРэТлХаЖЯРызгПеМфЙЙаЭЃЛ
(2)ЂйИљОнЮяжЪЕФЗжзгМфзїгУСІКЭЗжзгжЎМфЪЧЗёКЌгаЧтМќЗжЮіХаЖЯЃЛ
Ђкa.ИљОнМлВуЕчзгЖдЛЅГтРэТлШЗЖЈдгЛЏЗНЪНЃЛ
b.ЭЌвЛжїзхдЊЫиЕФЧтЛЏЮяжаЃЌКЌгаЧтМќЕФЧтЛЏЮяЗаЕуНЯИпЃЛ
c.ЬсЙЉЙТЕчзгЖдЕФдзгЪЧХфдзгЃЛ
d.CN-ЕФНсЙЙКЭЕЊЦјЗжзгЯрЫЦЃЌИљОнЕЊЦјЗжзгЕФЕчзгЪНХаЖЯЃЛ
(3)ЂйИљОнЯрЫЦЯрШмдРэШЗЖЈЗжзгЕФМЋадЃЛ
ЂкРћгУОљЬЏЗЈМЦЫуЃЛ
(4)РћгУОљЬЏЗЈМЦЫуИУОЇАћжаУОЁЂЧтдзгИіЪ§ЃЌдйИљОнV=![]() НјааМЦЫуЁЃ
НјааМЦЫуЁЃ
ЂйTiЪЧ22КХдЊЫиЃЌИљОнЙЙдьдРэПЩжЊЛљЬЌTiдзгКЫЭтЕчзгХХВМЪНЮЊЃК1s22s22p63s23p63d24s2ЃЌTiдзгЪЇШЅзюЭтВу2ИіЕчзгаЮГЩTi2+ЃЌдђTi2+ЛљЬЌЕФЕчзгХХВМЪНПЩБэЪОЮЊ1s22s22p63s23p63d2 (ЛђаДЮЊ[Ar]3d2)ЃЛ
ЂкBH4-жаBдзгМлВуЕчзгЖдЪ§ЮЊ4+![]() =4ЃЌЧвВЛКЌгаЙТЕчзгЖдЃЌЫљвдBH4-ЕФПеМфЙЙаЭЪЧе§ЫФУцЬхаЭЃЛ
=4ЃЌЧвВЛКЌгаЙТЕчзгЖдЃЌЫљвдBH4-ЕФПеМфЙЙаЭЪЧе§ЫФУцЬхаЭЃЛ
(2)ЂйдкИУЗДгІжаЩцМАЕФЮяжЪгаN2ЁЂH2ЁЂNH3ЃЌNH3ЗжзгжЎМфДцдкЧтМќЃЌЖјN2ЁЂH2ЗжзгжЎМфжЛДцдкЗжзгМфзїгУСІЃЌЫљвдNH3ЕФШлЗаЕуБШN2ЁЂH2ЕФИпЃЛгЩгкЯрЖдЗжзгжЪСПN2>H2ЃЌЮяжЪЕФЯрЖдЗжзгжЪСПдНДѓЃЌЗжзгМфзїгУСІОЭдНДѓЃЌЮяжЪЕФШлЗаЕуОЭдНИпЃЛЫљвдШ§жжЮяжЪЕФШлЕугЩЕЭЕНИпЕФЫГађЪЧH2< N2<NH3ЃЛ
Ђкa.NH3ЗжзгжаNдзгКЌга3ИіЙВгУЕчзгЖдКЭвЛИіЙТЕчзгЖдЃЌЫљвдЦфМлВуЕчзгЖдЪЧ4ЃЌВЩгУsp3дгЛЏЃЌaе§ШЗЃЛ
b.ЯрЭЌбЙЧПЪБЃЌАБЦјжаКЌгаЧтМќЃЌPH3жаВЛКЌЧтМќЃЌЫљвдNH3ЗаЕуБШPH3ИпЃЌbе§ШЗЃЛ
c.[Cu(NH3)4]2+РызгжаЃЌNдзгЬсЙЉЙТЕчзгЖдЃЌЫљвдNдзгЪЧХфЮЛдзгЃЌcе§ШЗЃЛ
d.CN-жаCЁЂNдзгЭЈЙ§Ш§ЖдЙВгУЕчзгЖдНсКЯЃЌЦфЕчзгЪНЮЊ![]() ЃЌdе§ШЗЃЛ
ЃЌdе§ШЗЃЛ
ЙЪКЯРэбЁЯюЪЧabcdЃЛ
(3)ЂйБНЁЂCS2ЖМЪЧЗЧМЋадЗжзгЃЌИљОнЯрЫЦЯрШмдРэЃЌгЩЗЧМЋадЗжзгЙЙГЩЕФШмжЪШнвзШмгкгЩЗЧМЋадЗжзгЙЙГЩЕФШмМСжаЃЌЫљвдC60ЪЧЗЧМЋадЗжзгЃЛ
ЂкРћгУОљЬЏЗЈжЊЃЌУПИіЬМдзгКЌгаІвМќЪ§ФПЮЊ![]() ЃЌдђ1mol C60ЗжзгжаЃЌКЌгаІвМќЪ§ФП=
ЃЌдђ1mol C60ЗжзгжаЃЌКЌгаІвМќЪ§ФП=![]() ЁС1molЁС60ЁСNA/mol=90NAЃЛ
ЁС1molЁС60ЁСNA/mol=90NAЃЛ
(4)ИУОЇАћжаУОдзгИіЪ§=![]() ЁС8+1=2ЃЌКЌгаЕФHдзгИіЪ§=2+4ЁС
ЁС8+1=2ЃЌКЌгаЕФHдзгИіЪ§=2+4ЁС![]() =4ЃЌдђОЇАћЕФЬхЛ§V=
=4ЃЌдђОЇАћЕФЬхЛ§V=![]() =
= g/cm3=
g/cm3=![]() g/cm3ЁЃ
g/cm3ЁЃ


 Н№дПГзЪдОэЯЕСаД№АИ
Н№дПГзЪдОэЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЮЊСЫБмУтNOЁЂNO2ЁЂN2O4ЖдДѓЦјЕФЮлШОЃЌГЃВЩгУNaOHШмвКНјааЮќЪеДІРэ(ЗДгІЗНГЬЪНЃК2NO2ЃЋ2NaOH===NaNO3ЃЋNaNO2ЃЋH2OЃЛNO2ЃЋNOЃЋ2NaOH===2NaNO2ЃЋH2O)ЁЃЯжгагЩa mol NOЁЂb mol NO2ЁЂc mol N2O4зщГЩЕФЛьКЯЦјЬхЧЁКУБЛV L NaOHШмвКЮќЪе(ЮоЦјЬхЪЃгр)ЃЌдђДЫNaOHШмвКЕФЮяжЪЕФСПХЈЖШЮЊ(ЁЁЁЁ)
A. ![]() molЁЄLЃ1 B.
molЁЄLЃ1 B. ![]() molЁЄLЃ1
molЁЄLЃ1
C. ![]() molЁЄLЃ1 D.
molЁЄLЃ1 D. ![]() molЁЄLЃ1
molЁЄLЃ1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈвЛЃЉЁЂЮЊСЫПЦбЇвћЪГЃЌСЫНтвЛаЉгыЪГЦЗЯрЙиЕФЛЏбЇжЊЪЖЪЧБивЊЕФЁЃ
ЃЈ1ЃЉгЭеЈЯКЬѕЁЂЪэЦЌЕШШнвзМЗЫщЕФЪГЦЗЃЌВЛвЫбЁгУецПеДќзАЃЌЖјгІВЩгУГфЦјДќзАЁЃЯТСаЦјЬхжаВЛгІИУГфШыЕФЪЧ___ЁЃ
A.ЕЊЦј B.ЖўбѕЛЏЬМ C.ПеЦј D.бѕЦј
ЃЈ2ЃЉЮЊЪЙвдУцЗлЮЊдСЯЕФУцАќЫЩШэПЩПкЃЌЭЈГЃгУЬМЫсЧтФЦзїЗЂХнМСЃЌвђЮЊЫќ___ЁЃ
ЂйШШЮШЖЈадВю ЂкдіМгЬ№ЮЖ ЂлВњЩњЖўбѕЛЏЬМ ЂмЬсЙЉФЦРызг
ЃЈ3ЃЉФмжБНгМјБ№ТШЛЏФЦКЭЦЯЬбЬЧСНжжЮДжЊХЈЖШШмвКЕФЗНЗЈЪЧ___ЁЃ
A.ЙлВьбеЩЋ B.ВтСПУмЖШ C.МгШШзЦЩе D.ЗжБ№ЮХЮЖ
ЃЈЖўЃЉЃЎЁЖЛЏбЇгыЩњЛюЁЗСМКУЕФЩњЬЌЛЗОГПЩвдЬсЩ§ЩњЛюжЪСПЁЃ
ЃЈ1ЃЉПеЦјжЪСПБЈИцЕФИїЯюжИБъПЩвдЗДгГГіИїЕиЕФПеЦјжЪСПЁЃЯТСаИїЯюжаФПЧАЮДСаШыЮвЙњПеЦјжЪСПБЈИцЕФЪЧ___(ЬюзжФИ)ЁЃ
aЃЎSO2 bЃЎNO2 cЃЎCO2 dЃЎPM2.5
ЃЈ2ЃЉРЌЛјгІЗжРрЪеМЏЁЃвдЯТЮяжЪгІЗХжУгкЬљгаБъжОРЌЛјЭВЕФЪЧ___(ЬюзжФИ)ЁЃ
![]()
aЃЎЗЯЕчГи bЃЎЗЯТСжЦЕФвзРЙо cЃЎНЈжўЙЬЦњЮяЁЂдќЭС
ЃЈ3ЃЉНќШеЃЌЙЋАВЛњЙиГЩЙІЦЦЛёСЫвЛЦ№ЬиДѓРћгУЁАЕиЙЕгЭЁБжЦЪлЪГгУгЭАИМўЁЃзлКЯРћгУЁАЕиЙЕгЭЁБЕФвЛжжЗНЗЈЃЌЭЈГЃНЋЁАЕиЙЕгЭЁБНјааМђЕЅМгЙЄЬсДПКѓЃЌЫЎНтЗжРыПЩЛёШЁ___КЭ___(ЬюУћГЦ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвдЯТЖд298KЪБpHЮЊ9ЕФKOHШмвКгыpHЮЊ9ЕФ![]() ШмвКжагЩЫЎЕчРыГіЕФ
ШмвКжагЩЫЎЕчРыГіЕФ![]() ЕФБШНЯжаЃЌе§ШЗЕФЪЧ
ЕФБШНЯжаЃЌе§ШЗЕФЪЧ![]()
A.СНепЯрЕШB.ЧАепЪЧКѓепЕФ![]() БЖ
БЖ
C.КѓепЪЧЧАепЕФ![]() БЖD.ЮоЗЈБШНЯ
БЖD.ЮоЗЈБШНЯ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвдЯТ6жжЫЕЗЈжае§ШЗЕФЪ§ФПЪЧ
![]() дкЯѕЫсеєЦјЕФЗеЮЇжаеєИЩ
дкЯѕЫсеєЦјЕФЗеЮЇжаеєИЩ![]() ШмвКЛсЕУЕН
ШмвКЛсЕУЕН![]() ЙЬЬх
ЙЬЬх
![]() ШмНтЖШКЭ
ШмНтЖШКЭ![]() вЛбљжЛгыЮТЖШгаЙи
вЛбљжЛгыЮТЖШгаЙи
![]() ЪЙгУОЋУмpHЪджНВтГі84ЯћЖОвКЕФpHЮЊ
ЪЙгУОЋУмpHЪджНВтГі84ЯћЖОвКЕФpHЮЊ![]()
![]() Щ§ЮТЖдгкЧПЫсЁЂЧПМюpHВЛЗЂЩњБфЛЏЃЌШѕЫсЩ§ЮТpHБфаЁ
Щ§ЮТЖдгкЧПЫсЁЂЧПМюpHВЛЗЂЩњБфЛЏЃЌШѕЫсЩ§ЮТpHБфаЁ
![]() ЕЮЖЈАБЫЎгУМзЛљГШзіжИЪОМСаЇЙћИќКУ
ЕЮЖЈАБЫЎгУМзЛљГШзіжИЪОМСаЇЙћИќКУ
![]() ЪЕбщЪвжЦЧтЦјЃЌЮЊМгПьЧтЦјЕФЩњГЩЫйТЪЃЌПЩЯђЯЁСђЫсжаЕЮМгЩйСП
ЪЕбщЪвжЦЧтЦјЃЌЮЊМгПьЧтЦјЕФЩњГЩЫйТЪЃЌПЩЯђЯЁСђЫсжаЕЮМгЩйСП![]() ШмвКЃЎ
ШмвКЃЎ
A.1B.2C.3D.ШЋВПе§ШЗ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШМУКбЬЦјжаКЌгаДѓСПNOxЁЂCO2ЁЂCOКЭSO2ЃЌОДІРэПЩЛёЕУживЊЕФЛЏЙЄдСЯЁЃ
(1)гУCH4ДпЛЏЛЙдNOxПЩвдЯћГ§ЕЊбѕЛЏЮяЕФЮлШОЁЃ
CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g)ЁЁІЄH1=Ѓ574.0 kJЁЄmolЃ1ЁЁ
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g)ЁЁІЄH2=ЃЋ1 160.0 kJЁЄmolЃ1ЁЁ
ЂйЗДгІCH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(g) ІЄH3=___________kJЁЄmolЃ1ЁЃ
ЂкШєЗДгІжаЛЙдNOxжСN2ЃЌЯћКФБъзМзДПіЯТ4.48L CH4ЃЌдђЗДгІЙ§ГЬжазЊвЦЕФЕчзгзмЪ§ЮЊ_____ЁЃ
(2)РћгУбЬЦјжаЗжРыЫљЕУЕФCO2ЁЂCOгыH2АДвЛЖЈБШР§ЛьКЯдкДпЛЏМСЕФзїгУЯТКЯГЩМзДМЃЌЗЂЩњЕФжївЊЗДгІШчЯТЃК
ЗДгІ1: CO(g)+2H2(g)=CH3OH(g)ІЄH1=Ѓ99.0 kJЁЄmolЃ1
ЗДгІ2: CO2(g)+3H2(g)=CH3OH(g)ЃЋH2O(g)ІЄH2=ЃЋ483.0 kJЁЄmolЃ1
ЗДгІ3: CO2(g)+H2(g)=CO(g)+H2O(g)ІЄH3=ЃЋ384.0 kJЁЄmolЃ1
ЗДгІЬхЯЕжаCOЦНКтзЊЛЏТЪ(ІС)гыЮТЖШКЭбЙЧПЕФЙиЯЕШчЭМЫљЪОЁЃ
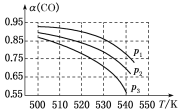
ЂйІС(CO)ЫцЮТЖШЩ§ИпЖјМѕаЁЕФдвђЪЧ____________________ЁЃ
ЂкЭМжаЕФp1ЁЂp2ЁЂp3гЩДѓЕНаЁЕФЫГађЮЊ__________________ЁЃ
(3)бЧТШЫсФЦ(NaClO2)КЭДЮТШЫсФЦ(NaClO)ЛьКЯвКзїЮЊИДКЯЮќЪеМСПЩЭбГ§бЬЦјжаЕФNOxЁЂSO2ЃЌЪЙЦфзЊЛЏЮЊNO3-ЁЂSO42-ЁЃ
ЂйаДГіNOгыNaClO2дкМюадЛЗОГжаЗДгІЕФРызгЗНГЬЪНЃК________________ЁЃ
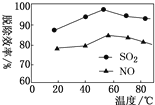
ЂкЯТЭМБэЪОдквЛЖЈЬѕМўЯТЮТЖШгыИДКЯЮќЪеМСЖдбЬЦјжаSO2ЁЂNOЭбГ§аЇТЪЕФЙиЯЕЁЃЭМжаSO2БШNOЭбГ§аЇТЪИпЕФдвђПЩФмЪЧЃК____________________ЁЃ
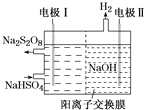
ЂлДгИДКЯЮќЪеМСЮќЪебЬЦјКѓЕФЗЯвКжаПЩЛиЪеЕУЕНNaHSO4ЃЌЕЭЮТЕчНтNaHSO4ЫЎШмвКПЩжЦБИЙЄвЕЩЯГЃгУЕФЧПбѕЛЏМСNa2S2O8ЃЌдРэШчЭМЫљЪОЁЃЕчНтЪБЕчМЋЂёЕФЕчМЋЗДгІЪНЮЊ______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвЛЖЈЮТЖШЯТЃЌКуШнУмБеШнЦїФкФГвЛЗДгІЬхЯЕжаMЁЂNЕФЮяжЪЕФСПЫцЗДгІЪБМфБфЛЏЕФЧњЯпШчгвЭМЫљЪОЃЌЯТСаа№Ъіе§ШЗЕФЪЧЃЈ ЃЉ

A. ИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ2M![]() N
N
B. t1ЪБNЕФХЈЖШЪЧMХЈЖШЕФ2БЖ
C. t2ЪБе§ЁЂФцЗДгІЫйТЪЯрЕШЃЌЗДгІДяЕНЦНКтзДЬЌ
D. t3ЪБе§ЗДгІЫйТЪДѓгкФцЗДгІЫйТЪ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПNaЁЂCuЁЂOЁЂSiЁЂSЁЂClЪЧГЃМћЕФСљжждЊЫиЁЃ
(1)NaЮЛгкдЊЫижмЦкБэЕк____жмЦкЕк____зхЃЛSЕФЛљЬЌдзгКЫЭтга________ИіЮДГЩЖдЕчзгЃЛSiЕФЛљЬЌдзгКЫЭтЕчзгХХВМЪНЮЊ__________ЁЃ
(2)гУЁА>ЁБЛђЁА<ЁБЬюПеЃК
ЕквЛЕчРыФм | РызгАыОЖ | ШлЕу | Ысад |
Si____S | O2-____Na+ | NaCl____Si | H2SO4____HClO4 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаРызгЗНГЬЪНФмгУРДНтЪЭЯргІЪЕбщЯжЯѓЕФЪЧЃЈ ЃЉ
ЪЕбщЯжЯѓ | РызгЗНГЬЪН | |
A | ЯђЧтбѕЛЏУОаќзЧвКжаЕЮМгТШЛЏяЇШмвКЃЌГСЕэШмНт |
|
B | ЯђЗаЫЎжаЕЮМгБЅКЭТШЛЏЬњШмвКЕУЕНКьКжЩЋвКЬх |
|
C | ЖўбѕЛЏСђЪЙЫсадИпУЬЫсМиШмвКЭЪЩЋ |
|
D | бѕЛЏбЧЬњШмгкЯЁЯѕЫс |
|
A. AB. BC. CD. D
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com