
����Ŀ��BaCl2��xH2O����;�㷺�Ļ���������Ʒ���ҹ�Ŀǰ��Ҫ�����������(������Mg2����Fe3����)��Ӧ����BaCl2��xH2O������������ͼ��ʾ����ش�
��֪������ʱKsp[Mg(OH)2]=1.8��10��11��Ksp[Fe(OH)3]=4.0��10��38
��1����ӦI�����ɵ�H2S��������ˮ���գ�һ����������������Һ��ͨ�˿������ֿɵõ�������ʹ����Һ������������Ӧ�Ļ�ѧ����ʽΪ_________��
��2�������Ȼ�����Һ�к�����(H2S��HS����)Ӱ���Ʒ�������ɹ���Ԥ�Ⱥ�Ŀ���������Ԥ�ȿ�����Ŀ����_________������A����Ҫ�ɷ���_________��
��3���ȿ�������ʱ���в���HS��ת��ΪS2O32����ʹ��Ʒ�Բ��ܴﵽ����Ҫ�������ữ�����ữ����ʱ�����ӷ���ʽΪ_________��
��4������ʱ��ΪʹMg2����Fe3����ȫ����(����Һ������Ũ��С��1��l0��5mol![]() ʱ��Ϊ��������ȫ����)��Ӧ����Һ��pH����_________(ֻ����ʽ)���ϡ�
ʱ��Ϊ��������ȫ����)��Ӧ����Һ��pH����_________(ֻ����ʽ)���ϡ�
��5��ʵ���Ҳⶨ��Ʒ��x�IJ������£�
��ȷ��ȡ12.23 g BaCl2��xH2O��Ʒ������l00 mLϡ��������ܽ⣻
���߽��裬����μ���0.lmol![]() H2SO4��Һ����BaSO4��ȫ���������ˣ�������ϴ�ӳ���2��3�Σ�
H2SO4��Һ����BaSO4��ȫ���������ˣ�������ϴ�ӳ���2��3�Σ�
����������ָ�������������Ϊ11.65 g������BaSO4�����Ƿ�ϴ�Ӹɾ��ķ�����_______��������x����ֵΪ_________��
���𰸡�2(NH4)2S ��O2��2H2O = 4NH3��H2O��2S�� ���½�ʹ������ܽ�ȼ�С�����ڴ���H2S S(����) S2O32����2H��=S����SO2����H2O -lg![]() ȡ���һ��ϴ��Һ�����������ữ����������Һ����������ϴ�Ӹɾ� 2
ȡ���һ��ϴ��Һ�����������ữ����������Һ����������ϴ�Ӹɾ� 2
��������
�����������������Mg2+��Fe3+�ȣ���Ӧ����BaCl2xH2O������������Mg2+��Fe3+�ȣ������ᷴӦ�����������壬�����Ȼ�����Һ�к����H2S��HS-�ȣ�Ӱ���Ʒ�������ɹ���Ԥ�Ⱥ�Ŀ������������ⱻ�����������ɳ���AΪ�����������Һ������ҺPH��ȥMg2+��Fe3+���õ�����BΪMg��OH��2��Fe��OH��3���õ��Ȼ�����Һ����Ũ���õ��Ȼ������壬���еõ���ĸҺѭ��ʹ�ã��ݴ˷�������
��1����ӦI�����ɵ�H2S��������ˮ���գ�������泥�����������������Ӧ���ɵõ�������ʹ����Һ������˵���������˰�ˮ��������Ӧ�Ļ�ѧ����ʽΪ2(NH4)2S ��O2��2H2O = 4NH3��H2O��2S����
��2�������Ȼ�����Һ�к�����(H2S��HS����)Ӱ���Ʒ�������ɹ���Ԥ�Ⱥ�Ŀ���������Ԥ�ȿ�����Ŀ�������½�ʹ������ܽ�ȼ�С�������ڴ���H2S�������ȿ����Ĺ������̳�Ϊ�������Գ���A����Ҫ�ɷ���S��
��3��S2O32����������Һ�лᷢ�������绯��Ӧ������S��SO2�������ữ����ʱ�����ӷ���ʽΪS2O32����2H��=S����SO2����H2O��
��4������Ksp[Mg(OH)2]=1.8��10��11��c(OH��)2��10��5=1.8��10��11�����c(OH��)=![]() ��10��3mol/L������ Ksp[Fe(OH)3]=4.0��10��38��c(OH��)3��10��5=4.0��10��32�����c(OH��)=
��10��3mol/L������ Ksp[Fe(OH)3]=4.0��10��38��c(OH��)3��10��5=4.0��10��32�����c(OH��)=![]() ��10��9mol/L��˵��Mg(OH)2�ܽ�Ƚϴ�Mg(OH)2��ȫ�γɳ�������pH����pH=-lg
��10��9mol/L��˵��Mg(OH)2�ܽ�Ƚϴ�Mg(OH)2��ȫ�γɳ�������pH����pH=-lg![]() ��
��
��5�����ᱵ�������������г�������������BaSO4�����Ƿ�ϴ�Ӹɾ������Լ���ϴ��Һ���Ƿ���Cl�����ɣ����巽����ȡ���һ��ϴ��Һ�����������ữ����������Һ����������ϴ�Ӹɾ�������Ba2���غ�ɵù�ϵʽΪ
BaCl2��xH2O����BaSO4
208��18x 233
12.23 11.65
��� (208��18x):233=12.23 : 11.65 ���x=2��


 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���ȼ����ؿ���������������Ĺ�����Դ���ȼ���������Ϊ����ʵĹ���LiCl-KCl������������ں�ؼ���˲��������ܡ��õ���ܷ�ӦΪ��PbSO4+2LiCl+Ca=CaCl2+Li2SO4+Pb�������й�˵����ȷ���ǣ� ��

A.������Ӧʽ��PbSO4+2e-+2Li+==LiSO4+Pb
B.�ŵ�����У������ɸƵ缫��������Ǧ�缫
C.�����£���ع���ÿת��0.1mol���ӣ�����������10.35gPb
D.�ŵ������,Cl-���ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʵ���У���Ӧ������ͽ��۶���ȷ�������߾��������ϵ���ǣ� ��
ѡ�� | ʵ�� | ���� | ���� |
A | ������̼��Ʒ�ĩ���뵽����NH4Cl��Һ�� | �������壬��ĩ�ܽ� | NH4Clˮ��ʹ��Һ������ |
B | ��BaSO4�����ĩ���뱥��Na2CO3��Һ�У����裬���ˣ�ϴ�ӣ��������м���ϡ���� | �������壬���������ܽ� | Ksp(BaCO3)<Ksp(BaSO4) |
C | ����ɫ����ͭ��ĩ���и��¼��� | ��ɫ��ɺ�ɫ | CuO�ֽ�����ͭ���� |
D | ��ij��Һ�μӹ�����ϡ���� | �д̼�����ζ�����������Һ�г��ֳ��� | ��Һ��һ������S2-��SO32- |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
��1��NH4Al(SO4)2������ˮ������������_____________________________(�ñ�Ҫ�Ļ�ѧ������������˵��)��
��2����ͬ�����£�0.1 mol��L��1NH4Al(SO4)2��c(![]() )________0.1 mol��L��1NH4HSO4��c(
)________0.1 mol��L��1NH4HSO4��c(![]() )��(����������������������������)
)��(����������������������������)
��3������ʽ��ʾ0.1 mol��L��1NH4Al(SO4)2��Һ�и�����Ũ�ȹ�ϵ____________��
��4����ͼ��0.1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
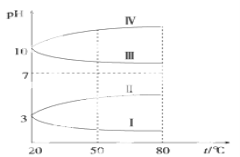
���з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯��������________(��д��ĸ)������pH���¶ȱ仯��ԭ����_____________________________��
��5������ʱ����100 mL 0.1 mol��L��1HCl��Һ�еμ�0.1 mol��L��1��ˮ���õ���ҺpH�백ˮ����Ĺ�ϵ������ͼ��ʾ��

���Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������____________��
����b�㣬��Һ�и�����Ũ���ɴ�С������˳����__________________;
��д��a������Һ��������ʽ�ľ�ȷ��������ܽ��Ƽ��㣩��
c��Cl-��- c��NH4+��=____________��c��H+��- c��NH3��H2O��=____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ҫ�Ļ�����Ʒ֮һ���о��ϳɰ���Ӧ������Ҫ���塣
��1����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��![]() ��
��![]() ��
��![]() ��д����N2��H2Ϊԭ�Ϻϳ�NH3���Ȼ�ѧ����ʽ______��
��д����N2��H2Ϊԭ�Ϻϳ�NH3���Ȼ�ѧ����ʽ______��
��2��ijС���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬ʵ��������ͼ ��ʾ��
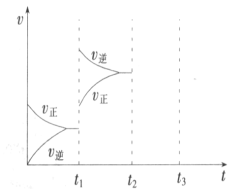
��t1ʱ�̸ı������Ϊ__________________��
��t2ʱ�̣���ѹ���뺤��,t3ʱ�̴ﵽƽ�⡣��ͼ�л���t2ʱ�̺�����ʱ仯ͼ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�绯��ʴ�Ǹ���������ʴ����Ҫԭ��ˮĤ������Ũ�Ƚ�Сʱ����Ҫ����________��ʴ���为����ӦʽΪ____________________��������ӦʽΪ______________________��
(2)���Ȼ�����Һ�������յõ��Ĺ���������______��(�ѧʽ����ͬ)����������Һ���ɵõ��Ĺ���������___________________ ��
(3)��CaSO4ˮ���ķ�����д����Ӧ�Ļ�ѧ����ʽ_________________��_________________���ȵĴ�����Һϴ��Ч�����õ�ԭ����_________________________��������ˮ�����ӷ���ʽ__________________��
(4)��2 mL 0.1 mol��L-1��NaCl��Һ�У�����2 mL 0.1 mol��L-1��AgNO3��Һ���ɹ۲쵽______________���˷�Ӧ�����ӷ���ʽΪ_________�����˻��Һ���ˣ���������2 mL 0.1 mol��L-1��KI��Һ�����裬�ɹ۲쵽____����Ӧ�����ӷ���ʽΪ________��
(5)����Ag2S(s)![]() 2Ag+(aq)+S2-(aq)����Ksp�ı���ʽΪ_______��
2Ag+(aq)+S2-(aq)����Ksp�ı���ʽΪ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������SO2�������У���ȷ���ǣ� ��
A. SO2��Ħ��������64g
B. 1 molSO2������������ԼΪ6.02��1023��
C. 1 molSO2��������64g/mol
D. ���³�ѹ�£�1 molSO2�����Ϊ22.4 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ4A(g)+3B(g)��2C(g)+D(g)����2min��B��Ũ�ȼ���0.6 molL-1���Դ˷�Ӧ���ʵı�ʾ��ȷ����( )
����A��ʾ�ķ�Ӧ������0.4 molL-1min-1
���ֱ���B��C��D��ʾ�ķ�Ӧ�������ֵΪ3��2��1
����2 minĩ�ķ�Ӧ���ʣ���B��ʾ��0.3 molL-1min-1
������2 min����B��ʾ�ķ�Ӧ���ʵ�ֵ����С�ģ���C��ʾ�ķ�Ӧ���ʵ�ֵ���������
A. �٢�
B. ��
C. ��
D. �ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�ֳ���ˮ����������ʳ���������Ѹɣ�����֮�⣬���ѻ���������ơ�
(1)��������֭���������ǵķ����ǣ������мӼ�������ԣ��ټ������Ƶ�Cu(OH)2�����ȣ���������________��
(2)��������ƹ����У�������ת��Ϊ�ƾ��Ĺ������£�����������л�ѧ����ʽ��C6H12O6(������)![]() 2_________+ 2 C2H5OH
2_________+ 2 C2H5OH
(3)���Ѿ��ܷⴢ������л���������ζ�����࣬����Ҳ����ͨ����ѧʵ�����Ʊ���ʵ���ҿ�����ͼ��ʾװ���Ʊ�����������

���Թ�a���������������Ļ�ѧ����ʽ��__________��
���Թ�b��ʢ�ŵ��Լ��DZ���____________��Һ��
��ʵ�鿪ʼʱ���Թ�b�еĵ��ܲ�����Һ���µ�ԭ����________��
����Ҫ������Թ�b�е�������������Ҫ�õ���������_______(����ĸ)��
A.��ͨ©�� B.��Һ©�� C.����©��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com